Dear friends, the following articles, written by a variety of doctors, journalists and medical researchers, illustrate some of the dangers to patients caused by the philosophy and entwined interests of the medical profession and chemical manufacturers.
Here is a link to a documentary on the use of prescription drugs: http://www.prescriptionthugs.com/
This Painful Syndrome Can Occur After Breast Cancer Surgeries, But Doctors Don’t Talk About ItBY THE BREAST CANCER SITE
Some women find that their pain actually worsens after mastectomy surgery. They experience intense burning pain in their chests or under their arms, sometimes accompanied by unbelievable itching. For 20 to 50 percent of these women, that pain never goes away. The pain is known as post-mastectomy pain syndrome.
While researchers don’t really know what causes PMPS, they assume that some form of nerve damage is to blame. Theories state that it may be caused by the formation of a neuroma, which is the abnormal growth of nerve tissue, or by a hematoma (or bruise) that somehow affects the nerves.
Women undergoing mastectomy or lumpectomy surgeries are typically told to expect various post-surgical side effects, including infection, build-up of fluids in the wound, and swelling of the arm or chest. However, very few women are warned in advance about PMPS, perhaps in part because not all doctors are aware of the condition. Nevertheless, 20 to 68 percent of mastectomy patients experience it, with the pain sometimes not starting until several months after their surgeries. However, it’s not just mastectomy patients that may experience it; even patients undergoing a lumpectomy or lymph node removal can develop this painful syndrome
PMPS pain can range from mild to severe. In more severe cases, it can lead to impaired movement and frozen shoulder. Stretching or the movements involved in household chores can make the pain worse.
Fortunately, PMPS pain is treatable. Treatment starts with NSAIDs such as ibuprofen or aspirin, then progresses to injectable steroids. Lidocaine, which is an anesthetic, can also be used for short-term relief of the pain. One of the most effective pain blockers is a combination of nerve blocker bupivacaine with dexamethasone, which is a steroid. This combination has been used for other post-surgical pain, but its first use for PMPS came in 2011. It appears to be effective for as long as six months at a time.
PMPS isn’t the only unexpected post-surgical side effect with potentially long-lasting consequences. Studies show that cancer surgery patients often experience PTSD as well.
Leading Cause of Death in Patients With Breast Cancer Posted: Friday, July 21, 2017
It may be surprising to some, but the leading cause of death in patients with breast cancer is heart disease.
“It’s no longer that they are diagnosed with breast cancer and they are dying of breast cancer. They are dying of heart disease,” said Sandra Cuellar, PharmD, BCOP, of the University of Illinois Hospital and Health Sciences System in Chicago, at the 13th Annual Conference of the Hematology/Oncology Pharmacy Association. Heart failure and other cardiotoxicities are occurring in women in their 40s and 50s after treatment with radiation, anthracyclines, and HER2-receptor antagonists, added Dr. Cuellar, who is Clinical Assistant Professor and Clinical Oncology Pharmacist at the University of Illinois.
However, if women receive cardioprevention within 2 months after cancer treatment, she noted, the cardiomyopathies may be mitigated or perhaps in some cases reversed. Furthermore, the change in the lifetime cumulative dose of anthracyclines—from between 400 and 500 mg/m2 to 250 mg/m2 or more—may help to mitigate some of the risks. “We have to retrain ourselves to this new threshold,” stated Dr. Cuellar. “We know we are going to cause cardiotoxicity, but there may be something we can do about it.”
Dr. Cuellar believes the cancer-cardiotoxicity statistics are changing the relationship between oncologists and cardiologists. With cardio-oncology emerging as a subspecialty, she recommends patients see a cardiologist before, during, and after treatment for cancer." https://jnccn360.org/brca/news/leading-cause-of-death-in-patients-with-breast-cancer/
Or, you can choose to heal yourself without surgery, radiation and chemotherapy and avoid being poisoned into heart disease.
Butylated hydroxyanisole — better known as BHA
— is “reasonably anticipated to be a human carcinogen” (a cancer-causing agent). According to the National Institute of Health, BHA in the diet has been found to consistently produce certain types of tumors in laboratory animals.
You can find it in (drum roll, please): potato chips, lard, butter, cereal, instant mashed potatoes,preserved meat, beer, baked goods, dry beverage and dessert mixes, chewing gum, and other foods. Oh, also: rubber, petroleum products, and, of course, wax food packaging. When fats and oils (in food, fats that are liquid a
re room temperature are called oils) are exposed to the air, oxidation occurs – the same process that causes old cars to rust. As previous Food Facts articles have mentioned, fats and oils have three long carbon chains. The more kinks in the chain, very generally speaking, the healthier and more fluid the fat is.
Unfortunately, when it comes to what makes a fat or oil rancid, the chemical bonds responsible for the kinks equate to a weakness in the fat's armor. Over time, oxygen in the air attacks the bond, which can transform the fat into a variety of chemicals, many of which smell foul and can be toxic.
Enter the antioxidants. When fatty or oily foods are treated with BHA, or its chemical cousin BHT (butylated hydroxytoluene), the preservatives occupy the attention of attacking oxygen molecules in a process chemists refer to as "scavenging free radicals." As a result, the food tastes better for longer. From http://www.livescience.com/36424-food-additive-bha-butylated-hydroxyanisole.html
"The synthetic phenolic antioxidants (e.g. BHT, BHA) added to human and animal food are able to lengthen the life of organisms ...In spite of their possible tumor-promoting properties they could not be considered overtly toxic." http://www.ncbi.nlm.nih.gov/pubmed/3053283
Dementia and Over the Counter Drugs
A new study out of the University of Washington provides the strong evidence that certain popular drugs may increase the risk for dementia in older adults. The drugs share some common mechanisms within key areas of the brain, but are used primarily as ingredients in over-the-counter sleep, cough and cold, and allergy medicines as well as in the treatment of an overactive bladder and depression.
The most commonly used drug linked to dementia was diphenhydramine, which is used in many popular products such as Benadryl, Nytol Sominex, Theraflu, Triaminic Allergy, plus many others. Also implicated where drugs containing chlorpheniramine (Aller-Chlor); oxybutynin (Ditropan) and tolterodine (Detrol) for overactive bladder; and the tricyclicantidepressants, such as doxepin or amitriptyline.
Background Data Acetylcholine is a critical brain chemical involved in the transmission of the nerve impulse and is especially important for proper memory and cognitive function. For example, Alzheimer’s disease is associated with a severe reduction in acetylcholine levels due to reduced activity of the enzyme that manufactures acetylcholine (choline acetyltransferase).
Given the link between low acetylcholine levels and poor brain function, including dementia, previous studies have linked drugs to reduced acetylcholine activity as well as mild cognitive impairment. These drugs include the ones mentioned in the introduction above. While discontinuation of the drugs is thought to reverse the mental deficit, there is evidence that anticholinergic drugs may produce permanent changes leading to irreversible dementia. These drugs are known to cause short-term drowsiness or confusion, which is included in the prescribing information, but the long-term effects these drugs have on mental function are generally not known by physicians or the people taking them.
Other drugs, like sedative hypnotic drugs (sleeping pills) and antihistamines have also been linked to an increased risk for dementia. All of these types of drugs, both prescription and over-the-counter, are used at an alarming rate by the elderly population putting them at significant risk for dementia.
New Data To evaluate whether cumulative anticholinergic use is associated with a higher risk for incident dementia, researchers examined medical records from 3,434 participants 65 years or older with no dementia at study entry. Initial recruitment occurred from 1994 through 1996 and from 2000 through 2003 and data through September 30, 2012 were also included in these analyses
Exposure to anticholinergic was determined from computerized pharmacy records. Cumulative exposure was updated as participants were followed up over a 10-year period. About 20% of the population was found to be using anticholinergic drugs.
During the evaluation period, 797 participants (23.2%) developed dementia with 637 of these (80%) developing Alzheimer disease. A 10-year cumulative dose-response relationship was observed for dementia and Alzheimer disease. In other words, the higher the cumulative anticholinergic use, the greater the increased risk for dementia. The highest risk threshold was taking the minimum daily effective dose of one the anticholinergic agents every day for 3 years.
Based upon these results, the authors of the study propose efforts to increase awareness among health care professionals and older adults about the risk of the use of these drugs over time. Even at low dosage or recommended levels chronic use of these drugs should be avoided.
The Dangers of NSAIDS
from WebMD
- Nonsteroidal anti-inflammatory drugs, known as NSAIDs, are found in aspirin, Advil, Motrin, Aleve, and other medications.
- Acetaminophen is found in Tylenol and a number of generic pain relievers.
Acetaminophen RisksAcetaminophen is considered safe if used according to the directions. However, taking more than the recommended dose can cause liver disease, liver failure, and death. Often, liver damage occurs before any symptoms show up. Shockingly, acetaminophen has replaced viral hepatitis as the leading cause of acute liver failure in the United States. The risk is greatest in people who already have liver problems, such as hepatitis or cirrhosis. But anyone who takes too much of the drug for too long faces danger.
Experts say that despite growing worries, OTC painkillers can be safe and effective, even for people with chronic pain. But it’s crucial to follow a few rules.
Rule #1 for Over-the-Counter Painkillers: Don’t Take Them Lightly“The first mistake people make,” says Penney Cowan, founder and executive director of the American Chronic Pain Association, “is thinking that if something is over-the-counter, it’s safe and they can do anything they want with it. These are potent drugs that can have severe side effects.”
Unfortunately, many people take them without a second thought. Philippe Berenger, MD, says, “We can ask patients what medicines they take. But they almost never mention over-the-counter pain meds unless we ask specifically, ‘Are you taking Tylenol or Excedrin?’” Berenger is a pain management specialist at the Cleveland Clinic Foundation.
“Patients end up compounding these drugs. They’re taking Tylenol without keeping track of how much. Then they take a course of a cold medicine with acetaminophen. Then their doctor prescribes a drug with acetaminophen in it. All of a sudden they’re at toxic doses,” says Berenger.
Genetically Modifying Humans Via Antibiotics?
Lisa Bloomquist
A new kind of antibiotic has been developed by researchers at Oregon State University. The new antibiotics are called PPMOs, which stand for peptide-conjugated phosphorodiamidate morpholino oligomers. They are “a synthetic analog of DNA or RNA that has the ability to silence the expression of specific genes.” The way that PPMO antibiotics will work is to, “specifically target the underlying genes of a bacterium.” In plain English, PPMOs will genetically modify bacteria.
This may not sound like a horrible thing on initial glance… However, bacteria and the other single-celled organisms that make up the human microbiome are intimate parts of each human being.
The healthy adult body hosts ten times as many microbial cells as human cells, including bacteria, archaea, viruses, and eukaryotic microbes resident on nearly every body surface. The metagenome carried collectively by these microbial communities dwarfs the human genome in size, and their influences on normal development, diet and obesity, immunity, and disease are under active research.
The average 200 pound human body contains 6 pounds of microbiome organisms, including several billion bacteria. These bacteria act symbiotically with us, helping to digest food, extract vitamins and other nutrients from food, regulate the immune system and even contribute to each individual’s personality. Per an article published in Molecular Psychology, “CNS neurotransmission can be profoundly disturbed by the absence of a normal gut microbiota.” (4) Multiple neurochemicals are produced by gut bacteria, including 95% of the serotonin in each human body . The health of each person’s microbiome is intimately connected to both their physical and the mental health.
The bacteria that compose our microbiome work so synergistically with our human cells that the difference between “us” and “the bacteria” is difficult to decipher. Where do “we” begin and “they” end? If all of the bacteria in a person’s microbiome were killed off, that person would die. Bacteria are an intimate and important part of “us.” In genetically modifying “them,” are we genetically modifying “us?” How could genetically modified bacteria affect the balance of the human microbiome? How could they affect the bodily systems that the microbiome controls? How could a GM bacteria adversely affect human health including personality and behavior?
One of many other things to consider is that mitochondria, the energy centers of our cells, are very similar in structure and design to bacteria. Mitochondrial DNA is also much more vulnerable to environmental toxins than the rest of the human DNA.
Could PPMOs (or other drugs that genetically modify bacteria) modify human mitochondria? If so, what are the consequences of having genetically modified mitochondria? One consequence is that humans truly would be genetically modified. Perhaps that should be taken into consideration before developing drugs that genetically modify bacteria.
…human mitochondrial DNA has been being altered and damaged by a certain class of antibiotics, fluoroquinolones, for years without anyone saying a peep.
Genetic Modification via Antibiotics is Already Occurring
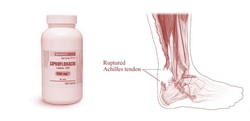
Fluoroquinolone antibiotics, more popularly known as Cipro (Ciprofloxacin), Levaquin (Levofloxacin), Avelox (Moxifloxacin), Floxin (Ofloxacin) and a few other less commonly used ones, are topoisomerase interrupters. They unravel bacterial DNA and lead to apoptosis, programmed cell death. They are used to treat urinary tract infections, sinus infections, bronchial infections, strep throat, etc. despite the fact that the side effects include psychosis and destruction of every tendon in the body. A side-effect that is lightly referred to as “tendinitis” on the warning label. (A more complete list of effects of fluoroquinolones can be found on www.ciproispoison.com. Multiple studies have shown that quinolones/fluoroquinolones attach to and change DNA. An example of another chemical that adducts to DNA is Agent Orange.
Some DNA tests performed on people who have experienced severe adverse reactions to fluoroquinolone antibiotics have shown that the quinolone/fluoroquinolone molecules have adducted to their human DNA, attaching to and changing their DNA into perpetuity. (As cells replicate, the altered DNA replicates too.) A DNA Adduct Mass Spectrogram Analysis showed that the quinolone/fluoroquinolone molecules had attached to every cell in the subjects’ bodies, not just the bacteria that make up their microbiome; the drug adducted to their DNA, to THEM.
They, along with thousands of other people who have had an adverse reaction to a fluoroquinolone, have been genetically modified by an antibiotic.
A large portion of those who have been genetically modified by a fluoroquinolone antibiotic have been subjected to irreversible damage to their DNA for no sensible reason at all. Fluoroquinolone antibiotics are given out to treat benign infections like sinus and urinary tract infections, that can be treated with other, safer antibiotics
So, if you’re wondering what happens when humans are genetically modified, the experiment is being conducted as you read this post. Please note that both Bayer (producer of Cipro and Avelox) and Johnson and Johnson (producer of Levaquin), and even the generic producers of these drugs, have very deep pockets.
A new kind of antibiotic has been developed by researchers at Oregon State University. The new antibiotics are called PPMOs, which stand for peptide-conjugated phosphorodiamidate morpholino oligomers. They are “a synthetic analog of DNA or RNA that has the ability to silence the expression of specific genes.” The way that PPMO antibiotics will work is to, “specifically target the underlying genes of a bacterium.” In plain English, PPMOs will genetically modify bacteria.
This may not sound like a horrible thing on initial glance… However, bacteria and the other single-celled organisms that make up the human microbiome are intimate parts of each human being.
The healthy adult body hosts ten times as many microbial cells as human cells, including bacteria, archaea, viruses, and eukaryotic microbes resident on nearly every body surface. The metagenome carried collectively by these microbial communities dwarfs the human genome in size, and their influences on normal development, diet and obesity, immunity, and disease are under active research.
The average 200 pound human body contains 6 pounds of microbiome organisms, including several billion bacteria. These bacteria act symbiotically with us, helping to digest food, extract vitamins and other nutrients from food, regulate the immune system and even contribute to each individual’s personality. Per an article published in Molecular Psychology, “CNS neurotransmission can be profoundly disturbed by the absence of a normal gut microbiota.” (4) Multiple neurochemicals are produced by gut bacteria, including 95% of the serotonin in each human body . The health of each person’s microbiome is intimately connected to both their physical and the mental health.
The bacteria that compose our microbiome work so synergistically with our human cells that the difference between “us” and “the bacteria” is difficult to decipher. Where do “we” begin and “they” end? If all of the bacteria in a person’s microbiome were killed off, that person would die. Bacteria are an intimate and important part of “us.” In genetically modifying “them,” are we genetically modifying “us?” How could genetically modified bacteria affect the balance of the human microbiome? How could they affect the bodily systems that the microbiome controls? How could a GM bacteria adversely affect human health including personality and behavior?
One of many other things to consider is that mitochondria, the energy centers of our cells, are very similar in structure and design to bacteria. Mitochondrial DNA is also much more vulnerable to environmental toxins than the rest of the human DNA.
Could PPMOs (or other drugs that genetically modify bacteria) modify human mitochondria? If so, what are the consequences of having genetically modified mitochondria? One consequence is that humans truly would be genetically modified. Perhaps that should be taken into consideration before developing drugs that genetically modify bacteria.
…human mitochondrial DNA has been being altered and damaged by a certain class of antibiotics, fluoroquinolones, for years without anyone saying a peep.
Genetic Modification via Antibiotics is Already Occurring
Fluoroquinolone antibiotics, more popularly known as Cipro (Ciprofloxacin), Levaquin (Levofloxacin), Avelox (Moxifloxacin), Floxin (Ofloxacin) and a few other less commonly used ones, are topoisomerase interrupters. They unravel bacterial DNA and lead to apoptosis, programmed cell death. They are used to treat urinary tract infections, sinus infections, bronchial infections, strep throat, etc. despite the fact that the side effects include psychosis and destruction of every tendon in the body. A side-effect that is lightly referred to as “tendinitis” on the warning label. (A more complete list of effects of fluoroquinolones can be found on www.ciproispoison.com. Multiple studies have shown that quinolones/fluoroquinolones attach to and change DNA. An example of another chemical that adducts to DNA is Agent Orange.
Some DNA tests performed on people who have experienced severe adverse reactions to fluoroquinolone antibiotics have shown that the quinolone/fluoroquinolone molecules have adducted to their human DNA, attaching to and changing their DNA into perpetuity. (As cells replicate, the altered DNA replicates too.) A DNA Adduct Mass Spectrogram Analysis showed that the quinolone/fluoroquinolone molecules had attached to every cell in the subjects’ bodies, not just the bacteria that make up their microbiome; the drug adducted to their DNA, to THEM.
They, along with thousands of other people who have had an adverse reaction to a fluoroquinolone, have been genetically modified by an antibiotic.
A large portion of those who have been genetically modified by a fluoroquinolone antibiotic have been subjected to irreversible damage to their DNA for no sensible reason at all. Fluoroquinolone antibiotics are given out to treat benign infections like sinus and urinary tract infections, that can be treated with other, safer antibiotics
So, if you’re wondering what happens when humans are genetically modified, the experiment is being conducted as you read this post. Please note that both Bayer (producer of Cipro and Avelox) and Johnson and Johnson (producer of Levaquin), and even the generic producers of these drugs, have very deep pockets.
Prescription Drug Deaths

"The CDC reports that from 1999 to 2004, unintentional poisoning death from prescription drugs, sleeping pills, antidepressants and tranquilizers grew 84 percent to 20,950 deaths, overtaking cocaine and heroin combined as the leading cause of lethal overdose.
The FDA compiled reports from 1998 to 2005 and finds that dangerous side effects and deaths from prescription and over-the-counter medications almost tripled to nearly 90,000 incidents.
Potent narcotic painkiller OxyContin was among the 15 drugs most often linked to death. Others include insulin, Vioxx, Remicade, and Paxil. Vioxx was removed from the market in 2004.
According to the Journal of the American Medical Association (JAMA) in 1998, a report finds that prescription drugs kill about 106,000 Americans each year – that’s three times as many as are killed by automobiles—making prescription drug death the fourth leading killer after heart disease, cancer and stroke.
Last year Journal of the American Medical Association puts death from all drugs, illegal and prescription, second only behind car accidents as a cause of death. The rise in deaths coincides with the direct marketing of prescription medication to the public. Prescription drug sales have soared nearly 500 percent since 1990". Read more: http://news.injuryboard.com/prescription-drug-deaths-soar.aspx?googleid=29488#ixzz1PFwaQ0i6
The FDA compiled reports from 1998 to 2005 and finds that dangerous side effects and deaths from prescription and over-the-counter medications almost tripled to nearly 90,000 incidents.
Potent narcotic painkiller OxyContin was among the 15 drugs most often linked to death. Others include insulin, Vioxx, Remicade, and Paxil. Vioxx was removed from the market in 2004.
According to the Journal of the American Medical Association (JAMA) in 1998, a report finds that prescription drugs kill about 106,000 Americans each year – that’s three times as many as are killed by automobiles—making prescription drug death the fourth leading killer after heart disease, cancer and stroke.
Last year Journal of the American Medical Association puts death from all drugs, illegal and prescription, second only behind car accidents as a cause of death. The rise in deaths coincides with the direct marketing of prescription medication to the public. Prescription drug sales have soared nearly 500 percent since 1990". Read more: http://news.injuryboard.com/prescription-drug-deaths-soar.aspx?googleid=29488#ixzz1PFwaQ0i6
Mixing Prescription Drugs

"Sometimes it takes "celebrity" to bring to light a national issue, in this case a chronic condition for many everyday Americans.
Heath Ledger's death on Jan. 22 was due to an accidental mixture of prescription drugs, New York City's Chief Medical Examiner has concluded.
The autopsy report on Ledger is now public record and counts six prescription drugs as the cause of his death including Oxycodone, Hydrocodone, Diazepam, Temazepam, Alprazolam, and Doxylamine.
It is not likely a single doctor prescribed all of the drugs but rather that they were obtained from numerous sources. It is suspected that the combination of drugs suppressed his respiratory system until Ledger stopped breathing. Ledger's family hopes his death shines a spotlight on a long under-reported issue – you are now more likely to die from prescription drugs than recreational ones." Read more: http://news.injuryboard.com/prescription-drug-deaths-soar.aspx?googleid=29488#ixzz1PFv82udL
Heath Ledger's death on Jan. 22 was due to an accidental mixture of prescription drugs, New York City's Chief Medical Examiner has concluded.
The autopsy report on Ledger is now public record and counts six prescription drugs as the cause of his death including Oxycodone, Hydrocodone, Diazepam, Temazepam, Alprazolam, and Doxylamine.
It is not likely a single doctor prescribed all of the drugs but rather that they were obtained from numerous sources. It is suspected that the combination of drugs suppressed his respiratory system until Ledger stopped breathing. Ledger's family hopes his death shines a spotlight on a long under-reported issue – you are now more likely to die from prescription drugs than recreational ones." Read more: http://news.injuryboard.com/prescription-drug-deaths-soar.aspx?googleid=29488#ixzz1PFv82udL
Synthetic Vitamins

""...about 99% of all C vitamin products on the market today are synthetically made ascorbic acid - or variations such as calcium ascorbate, magnesium ascorbate or potassium ascorbate. Despite marketing claims, these laboratory-produced powders like ascorbic acid are NOT natural C complex vitamin - and far from it. They are chemically synthesized molecules manufactured in a test tube and are often made from genetically modified corn sugar. These synthetic molecules mimic only one component of the multitude of life-supporting nutrient complexes found in real natural vitamin C complex. Although some people will go to great lengths to try to convince you that synthetic C such as ascorbic acid is the same as food source C complex, there is much evidence to the contrary...Taking synthetic ascorbic acid can make the body more acidic over time and steals from the body’s calcium reserves, since the body must release calcium to neutralize the synthetic ascorbic acid. Higher acidity is associated with poorer health. Over time, taking synthetic ascorbic acid products may have negative effects on your health. Don’t be fooled by products that advertise C products along with rose hips or acerola - they usually contain only token amounts of the “real” nutrients - you’re still getting mostly ascorbic acid or synthetic C. " http://www.healthy-vitamins-rx.com/html/vitamin-c-synthetic-natural.html
"Naturally occurring in citrus fruits, acerola cherries, rose hips and other fruits and vegetables, this vitamin comes in a package containing vitamin P factors such as bioflavonoids and rutin, vitamin K, vitamin J, various enzymes and coenzymes plus a small amount of ascorbic acid, the antioxidant of the complex. Vitamin C is rated according to the amount of ascorbic acid it contains. Ascorbic acid is not vitamin C, ascorbic acid is ascorbic acid, a fraction of the biologically utilizable natural vitamin C complex. Furthermore, most ascorbic acid on the market is produced synthetically.
In a study conducted by Dr. Victor Herbert, professor of medicine at the Mount Sinai School of Medicine in New York, and published in The New York Times, it was found that rather than reduce free radicals which lead to cell damage, synthetic C supplements promoted free radical generation. "The vitamin C supplements mobilizes harmless ferric iron stored in the body and converts it to harmful ferrous iron, which induces damage to the heart and other organs. Unlike the vitamin C naturally present in foods like orange juice, ascorbic acid as a vitamin C supplement is not an antioxidant, it's a redox agent - an antioxidant in some circumstances and a pro-oxidant in others," said Dr. Herbert.
According to The New York Times, reporting on an another study, a team of British pathologists at the University of Leicester studied 30 healthy men and women for six weeks, giving them 400 milligrams of vitamin C daily in the form of ascorbic acid. They found that at this level, vitamin C promoted damage to the DNA in these individuals.
Synthetic B vitamins have performed similarly. Writing in a Pennsylvania newspaper, a medical columnist who had been medical officer in a North Korean prisoner-of-war camp during the Korean conflict, found his fellow prisoners contracting Beriberi, a disease caused by a deficiency of Vitamin B. He obtained Thiamine Hydrochloride, a synthetic form of vitamin B, from the Red Cross, and administered it to the sickest men. No positive change was seen and the men continued to get worse. The guards suggested rice polish, a natural source of vitamin B, which he administered in small amounts. The Beriberi symptoms abated within a week." http://www.myhealthstore.com/index.php?p=whynat
Vitamin D
"Vitamin D toxicity is usually caused by megadoses of vitamin D supplements — not by diet or sun exposure. That's because your body regulates the amount of vitamin D produced by sun exposure, and even fortified foods don't contain large amounts of vitamin D.
The main consequence of vitamin D toxicity is a buildup of calcium in your blood (hypercalcemia), which can cause symptoms such as poor appetite, nausea and vomiting. Weakness, frequent urination and kidney problems also may occur. Treatment includes the stopping of excessive vitamin D intake. Your doctor may also prescribe intravenous fluids and medications, such as corticosteroids or bisphosphonates..." http://www.mayoclinic.com/health/vitamin-d-toxicity/AN02008
"In the 1930’s, seeing great profit potential in sunshine hormones, Big Pharma went to work manufacturing a copy cat. In that pursuit, they narrowed the scope of our sunshine hormones and postulated that it was a single isolate that was responsible for the vast, biological benefits of sunshine. At the same time, they disregarded the unique balance and protection mechanism built by the body to guard against toxicity. Once successful in designing their “Franken-chemical,” they launched a campaign to systematically contaminate our vitamin and food supply with it and make billions.
Turning Sunshine into a Drug
Today, Big Pharma cartels BASF and Hoffman La Roche are the largest manufacturers of the synthetic hormone isolate. To get the masses to swallow it, they erroneously named it “The Sunshine Vitamin,” AKA vitamin D. Consumers, stimulated by ads, couldn’t wait to start choking it down, so much that old ladies bragged about taking the “drug disguised as a vitamin” to their hairdressers. In reality though, it’s as close to being a sunshine hormone as a tootsie roll is to being chocolate, or a porno is to having sex. I’m not the only one pointing out this fact.
“Vitamin D is not really a vitamin,” wrote scientists for the New England Journal of Medicine. For something to be a vitamin, it should provide the body with an essential nutrient that it cannot make on its own, but requires for survival.
Since synthetic vitamin D is a drug, foreign to the body, and not required for survival, it’s technically a fraud – an impostor posing as a vitamin. It has “vitamin like” activity, which initially tricks the body into thinking the host of associated co-hormones is present. But this biological ruse proves to be devastating to the body over time.
Before the vitamin D scam was fed to consumers, the deadly “D” was fed to rodents as a means of eradicating the pesky creatures. In their report, “The Endocrine System,” the University of Colorado, reminds us, "Ingestion of milligram quantities of vitamin D over periods of weeks or months can be severely toxic to humans and animals. In fact, baits laced with vitamin D are used very effectively as rodenticides [rat poison]." This is in stark contrast to naturally produced sunshine vitamins, and it isn’t hard to understand.
Once swallowed, the copycat hormone bypasses our innate protective mechanisms and throws hormonal balance out of whack. This “plugs” the body with calcium and induces calcification, which leads to heart failure, kidney damage, and more. Since it’s a “cumulative poison,” people who take the recommended dose every day as vitamin D are saturating their fatty tissues, and at the same time, offsetting their God-given hormonal intelligence. Anyone eating and drinking foods “fortified” with vitamin D are at serious risk. Nutrition guru Dr. Gary Null learned this the hard way.
After sucking down his “Power Meal” loaded with the vitamin D supplement, New York Daily News reported that Dr. Null was hit with "excruciating fatigue" that left him urinating blood and unable to walk. Upon checking into the hospital, Null was told that he could have died from his synthetic vitamin D (rodenticide) “overdose” - like suicide in slow motion. Null is suing his manufacturer for putting too much vitamin D in his products. He misses the point though: There is no safe dose. Synthetic vitamin D is poisonous in any amount due to its ability to get crammed into fat cells and accumulate over periods of months (compared to 20 minutes when naturally produced), thereby disrupting hormone balance. Nobody has ever been poisoned by naturally produced sunshine hormones." http://www.newswithviews.com/Ellison/shane158.htm
"Naturally occurring in citrus fruits, acerola cherries, rose hips and other fruits and vegetables, this vitamin comes in a package containing vitamin P factors such as bioflavonoids and rutin, vitamin K, vitamin J, various enzymes and coenzymes plus a small amount of ascorbic acid, the antioxidant of the complex. Vitamin C is rated according to the amount of ascorbic acid it contains. Ascorbic acid is not vitamin C, ascorbic acid is ascorbic acid, a fraction of the biologically utilizable natural vitamin C complex. Furthermore, most ascorbic acid on the market is produced synthetically.
In a study conducted by Dr. Victor Herbert, professor of medicine at the Mount Sinai School of Medicine in New York, and published in The New York Times, it was found that rather than reduce free radicals which lead to cell damage, synthetic C supplements promoted free radical generation. "The vitamin C supplements mobilizes harmless ferric iron stored in the body and converts it to harmful ferrous iron, which induces damage to the heart and other organs. Unlike the vitamin C naturally present in foods like orange juice, ascorbic acid as a vitamin C supplement is not an antioxidant, it's a redox agent - an antioxidant in some circumstances and a pro-oxidant in others," said Dr. Herbert.
According to The New York Times, reporting on an another study, a team of British pathologists at the University of Leicester studied 30 healthy men and women for six weeks, giving them 400 milligrams of vitamin C daily in the form of ascorbic acid. They found that at this level, vitamin C promoted damage to the DNA in these individuals.
Synthetic B vitamins have performed similarly. Writing in a Pennsylvania newspaper, a medical columnist who had been medical officer in a North Korean prisoner-of-war camp during the Korean conflict, found his fellow prisoners contracting Beriberi, a disease caused by a deficiency of Vitamin B. He obtained Thiamine Hydrochloride, a synthetic form of vitamin B, from the Red Cross, and administered it to the sickest men. No positive change was seen and the men continued to get worse. The guards suggested rice polish, a natural source of vitamin B, which he administered in small amounts. The Beriberi symptoms abated within a week." http://www.myhealthstore.com/index.php?p=whynat
Vitamin D
"Vitamin D toxicity is usually caused by megadoses of vitamin D supplements — not by diet or sun exposure. That's because your body regulates the amount of vitamin D produced by sun exposure, and even fortified foods don't contain large amounts of vitamin D.
The main consequence of vitamin D toxicity is a buildup of calcium in your blood (hypercalcemia), which can cause symptoms such as poor appetite, nausea and vomiting. Weakness, frequent urination and kidney problems also may occur. Treatment includes the stopping of excessive vitamin D intake. Your doctor may also prescribe intravenous fluids and medications, such as corticosteroids or bisphosphonates..." http://www.mayoclinic.com/health/vitamin-d-toxicity/AN02008
"In the 1930’s, seeing great profit potential in sunshine hormones, Big Pharma went to work manufacturing a copy cat. In that pursuit, they narrowed the scope of our sunshine hormones and postulated that it was a single isolate that was responsible for the vast, biological benefits of sunshine. At the same time, they disregarded the unique balance and protection mechanism built by the body to guard against toxicity. Once successful in designing their “Franken-chemical,” they launched a campaign to systematically contaminate our vitamin and food supply with it and make billions.
Turning Sunshine into a Drug
Today, Big Pharma cartels BASF and Hoffman La Roche are the largest manufacturers of the synthetic hormone isolate. To get the masses to swallow it, they erroneously named it “The Sunshine Vitamin,” AKA vitamin D. Consumers, stimulated by ads, couldn’t wait to start choking it down, so much that old ladies bragged about taking the “drug disguised as a vitamin” to their hairdressers. In reality though, it’s as close to being a sunshine hormone as a tootsie roll is to being chocolate, or a porno is to having sex. I’m not the only one pointing out this fact.
“Vitamin D is not really a vitamin,” wrote scientists for the New England Journal of Medicine. For something to be a vitamin, it should provide the body with an essential nutrient that it cannot make on its own, but requires for survival.
Since synthetic vitamin D is a drug, foreign to the body, and not required for survival, it’s technically a fraud – an impostor posing as a vitamin. It has “vitamin like” activity, which initially tricks the body into thinking the host of associated co-hormones is present. But this biological ruse proves to be devastating to the body over time.
Before the vitamin D scam was fed to consumers, the deadly “D” was fed to rodents as a means of eradicating the pesky creatures. In their report, “The Endocrine System,” the University of Colorado, reminds us, "Ingestion of milligram quantities of vitamin D over periods of weeks or months can be severely toxic to humans and animals. In fact, baits laced with vitamin D are used very effectively as rodenticides [rat poison]." This is in stark contrast to naturally produced sunshine vitamins, and it isn’t hard to understand.
Once swallowed, the copycat hormone bypasses our innate protective mechanisms and throws hormonal balance out of whack. This “plugs” the body with calcium and induces calcification, which leads to heart failure, kidney damage, and more. Since it’s a “cumulative poison,” people who take the recommended dose every day as vitamin D are saturating their fatty tissues, and at the same time, offsetting their God-given hormonal intelligence. Anyone eating and drinking foods “fortified” with vitamin D are at serious risk. Nutrition guru Dr. Gary Null learned this the hard way.
After sucking down his “Power Meal” loaded with the vitamin D supplement, New York Daily News reported that Dr. Null was hit with "excruciating fatigue" that left him urinating blood and unable to walk. Upon checking into the hospital, Null was told that he could have died from his synthetic vitamin D (rodenticide) “overdose” - like suicide in slow motion. Null is suing his manufacturer for putting too much vitamin D in his products. He misses the point though: There is no safe dose. Synthetic vitamin D is poisonous in any amount due to its ability to get crammed into fat cells and accumulate over periods of months (compared to 20 minutes when naturally produced), thereby disrupting hormone balance. Nobody has ever been poisoned by naturally produced sunshine hormones." http://www.newswithviews.com/Ellison/shane158.htm
The following is a list of medications and nonprescription drugs that may cause impotence in men:
Antidepressants and other psychiatric medications:
- Amitriptyline (Elavil)
- Amoxapine (Asendin)
- Buspirone (Buspar)
- Chlordiazepoxide (Librium)
- Chlorpromazine (Thorazine)
- Clomipramine (Anafranil)
- Clorazepate (Tranxene)
- Desipramine (Norpramin)
- Diazepam (Valium)
- Doxepin (Sinequan)
- Fluoxetine (Prozac)
- Fluphenazine (Prolixin)
- Imipramine (Tofranil)
- Isocarboxazid (Marplan)
- Lorazepam (Ativan)
- Meprobamate (Equanil)
- Mesoridazine (Serentil)
- Nortriptyline (Pamelor)
- Oxazepam (Serax)
- Phenelzine (Nardil)
- Phenytoin (Dilantin)
- Sertraline (Zoloft)
- Thioridazine (Mellaril)
- Thiothixene (Navane)
- Tranylcypromine (Parnate)
- Trifluoperazine (Stelazine)
- Cimetidine (Tagamet)
- Dimenhydrinate (Dramamine)
- Diphenhydramine (Benadryl)
- Hydroxyzine (Vistaril)
- Meclizine (Antivert)
- Nizatidine (Axid)
- Promethazine (Phenergan)
- Ranitidine (Zantac)
- Atenolol (Tenormin)
- Bethanidine
- Bumetanide (Bumex)
- Captopril (Capoten)
- Chlorothiazide (Diuril)
- Chlorthalidone (Hygroton)
- Clonidine (Catapres)
- Enalapril (Vasotec)
- Furosemide (Lasix)
- Guanabenz (Wytensin)
- Guanethidine (Ismelin)
- Guanfacine (Tenex)
- Haloperidol (Haldol)
- Hydralazine (Apresoline)
- Hydrochlorothiazide (Esidrix)
- Labetalol (Normodyne)
- Methyldopa (Aldomet)
- Metoprolol (Lopressor)
- Nifedipine (Adalat, Procardia)
- Phenoxybenzamine (Dibenzyline)
- Phentolamine (Regitine)
- Prazosin (Minipress)
- Propranolol (Inderal)
- Reserpine (Serpasil)
- Spironolactone (Aldactone)
- Triamterene (Maxzide)
- Verapamil (Calan)
Parkinson's disease medications:
Biperiden (Akineton)
- Bromocriptine (Parlodel)
- Levodopa (Sinemet)
- Procyclidine (Kemadrin)
- Trihexyphenidyl (Artane)
- Benztropine (Cogentin)
- Antiandrogens (Casodex, Flutamide, Nilutamide)
- Busulfan (Myleran)
- Cyclophosphamide (Cytoxan)
- Ketoconazole
- LHRH agonists (Lupron, Zoladex)
- Aminocaproic acid (Amicar)
- Atropine
- Clofibrate (Atromid-S)
- Cyclobenzaprine (Flexeril)
- Cyproterone
- Digoxin (Lanoxin)
- Disopyramide (Norpace)
- Estrogen
- Finasteride (Propecia, Proscar, Avodart)
- Furazolidone (Furoxone)
- H2 blockers (Tagamet, Zantac, Pepcid)
- Indomethacin (Indocin)
- Lipid-lowering agents
- Licorice
- Metoclopramide (Reglan)
- NSAIDs (Ibuprofen, etc.)
- Orphenadrine (Norflex)
- Prochlorperazine (Compazine)
- Pseudoephedrine (Sudafed)
- Codeine
- Fentanyl (Innovar)
- Hydromorphone (Dilaudid)
- Meperidine (Demerol)
- Methadone
- Morphine
- Oxycodone (Oxycontin, Percodan)
Vioxx
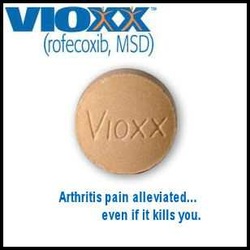
Recall that in April of 2002 the FDA added new warnings to labels of Vioxx about the increased risk of heart attack. After months of negative publicity, the non-steroidal anti-inflammatory COX-2 inhibitor drug Vioxx was finally recalled in September of 2004.
Whistleblower Dr. David Graham, in testimony before the US Senate, estimated 88,000 to 139,000 Americans experienced heart attacks as a side effect from the drug, and 30 to 40 percent of these died. That would be an estimated 27,000 to 55,000 preventable deaths attributed to Vioxx.
Vioxx at its peak was being taken by 20 million Americans. In 2003 sales of Vioxx totaled about $2.5 billion. Vioxx prescriptions were 19,959,000 in 2003 and 13,994,000 in 2004, a decline of about 6 million prescriptions (about a 30% drop). (Source: IMS Health) Nobody is saying it, but it looks like Vioxx did kill many thousands of Americans. from http://www.lewrockwell.com/sardi/sardi53.html
How Are Drugs Like Vioxx Approved by the FDA? "The most common problems with in house drug testing ran or funded by drug companies results from that profiting from favorable results—what happens to patients is lost in the rush for profits.
Most commonly the comparison is to a placebo, so all that is need to show is that the drug works, rather than works better than alternatives on the market. Secondly tests are run in ways that are designed for FDA approval. For example, healthy young people are enrolled in the study—when possible—and thus the incidents of side effects are much lower. Also tests are generally run for six weeks, again reducing the risk of side effects which might take months or years to become statistically significant.
The drug industry has yet to come up with a better NSAID than aspirin. As for avoiding gastrointestinal problems, one of their selling ploys, they generally test uncoated aspirin against a coated drug such as ibuprofen". http://healthfully.org/mdcrapm/id18.html
pril 19, 2010 by Rob McCaleb
Filed under All, Opinion and Comment, Politics, Top Stories
Research for Vioxx and Celebrex
Another black eye for the so-called “ethical pharmaceutical” business. In what is being called “the biggest research fraud in medical history,” a member of Pfizer’s “speaker bureau” has pled guilty to fabricating dozens of drug studies. Dr. Scott Reuben, working on a $75,000 grant from Pfizer produced a research study on their drug Celebrex. Naturally, the drug was found to be remarkably effective against pain. Well, except there were no patients in the study. It was completely fabricated.
This isn’t a first for the good (for business) doctor. The peer-reviewed (and shame on the reviewers) journal Anesthesia and Analesia had to retract 10 papers authored by Reuben. Another 21 Reuben articles were apparently also fabricated according to London’s The Day. Reuben received nearly a half million from Pfizer, which I guess he has to give back, and possibly pay a $250,000 fine on top of that. Maybe even jail time.
But for Pfizer and the other companies that got rave reviews from “ethical research,” nothing. Bextra and Vioxx were also beneficiaries of Reuben’s fairy tale “research.” But these drug companies are not treated as conspirators. They’re “victims” of this fraud. Coverage in The Day fills in the details. Reuben’s studies, five of which were funded by Pfizer, had bolstered claims about the post-surgery effectiveness of such painkillers as Pfizer’s Celebrex and Merck’s Vioxx. Reuben’s attorneys said a bipolar disorder with “alternating periods of mania and depression fueled his misconduct.”
Oh poor guy. I’m sure we can all understand how depression could make someone want to fake dozens of scientific articles. No, we can’t. That’s a BS defense. Meanwhile, these bogus “research studies” have been used to bolster claims of effectiveness for Celebrex and other drugs, as the public is fed the fiction that we have the best research in the world and the safest and most effective drugs. Yes, we have the best research results money can buy, and the best “approving for dollars” system too.
This corporate medical/scientific corruption hurts us all, and the media is only too happy to trot out the results of these “studies” while lapping up billions in pharma advertising.
A year earlier, Constantine Kostas admitted that of the 85 subjects in his clinical trial of Cipro, only 15 had actually been given Cipro. I suppose it hardly matters, since Kostas also faked the results of lab tests and examinations that never took place.
All of this is especially irksome to those of us who have endured Big Pharma’s war on herbs and supplements. How many of the so-called “research studies” on herbs funded by big pharma are also bogus? It’s easy to find such “studies” in which the patients chosen were inappropriate, or the protocol designed in ways that appear destined to fail. Indeed, “failure” of an alternative medicine is exactly what the drug companies want to see.
Whistleblower Dr. David Graham, in testimony before the US Senate, estimated 88,000 to 139,000 Americans experienced heart attacks as a side effect from the drug, and 30 to 40 percent of these died. That would be an estimated 27,000 to 55,000 preventable deaths attributed to Vioxx.
Vioxx at its peak was being taken by 20 million Americans. In 2003 sales of Vioxx totaled about $2.5 billion. Vioxx prescriptions were 19,959,000 in 2003 and 13,994,000 in 2004, a decline of about 6 million prescriptions (about a 30% drop). (Source: IMS Health) Nobody is saying it, but it looks like Vioxx did kill many thousands of Americans. from http://www.lewrockwell.com/sardi/sardi53.html
How Are Drugs Like Vioxx Approved by the FDA? "The most common problems with in house drug testing ran or funded by drug companies results from that profiting from favorable results—what happens to patients is lost in the rush for profits.
Most commonly the comparison is to a placebo, so all that is need to show is that the drug works, rather than works better than alternatives on the market. Secondly tests are run in ways that are designed for FDA approval. For example, healthy young people are enrolled in the study—when possible—and thus the incidents of side effects are much lower. Also tests are generally run for six weeks, again reducing the risk of side effects which might take months or years to become statistically significant.
The drug industry has yet to come up with a better NSAID than aspirin. As for avoiding gastrointestinal problems, one of their selling ploys, they generally test uncoated aspirin against a coated drug such as ibuprofen". http://healthfully.org/mdcrapm/id18.html
pril 19, 2010 by Rob McCaleb
Filed under All, Opinion and Comment, Politics, Top Stories
Research for Vioxx and Celebrex
Another black eye for the so-called “ethical pharmaceutical” business. In what is being called “the biggest research fraud in medical history,” a member of Pfizer’s “speaker bureau” has pled guilty to fabricating dozens of drug studies. Dr. Scott Reuben, working on a $75,000 grant from Pfizer produced a research study on their drug Celebrex. Naturally, the drug was found to be remarkably effective against pain. Well, except there were no patients in the study. It was completely fabricated.
This isn’t a first for the good (for business) doctor. The peer-reviewed (and shame on the reviewers) journal Anesthesia and Analesia had to retract 10 papers authored by Reuben. Another 21 Reuben articles were apparently also fabricated according to London’s The Day. Reuben received nearly a half million from Pfizer, which I guess he has to give back, and possibly pay a $250,000 fine on top of that. Maybe even jail time.
But for Pfizer and the other companies that got rave reviews from “ethical research,” nothing. Bextra and Vioxx were also beneficiaries of Reuben’s fairy tale “research.” But these drug companies are not treated as conspirators. They’re “victims” of this fraud. Coverage in The Day fills in the details. Reuben’s studies, five of which were funded by Pfizer, had bolstered claims about the post-surgery effectiveness of such painkillers as Pfizer’s Celebrex and Merck’s Vioxx. Reuben’s attorneys said a bipolar disorder with “alternating periods of mania and depression fueled his misconduct.”
Oh poor guy. I’m sure we can all understand how depression could make someone want to fake dozens of scientific articles. No, we can’t. That’s a BS defense. Meanwhile, these bogus “research studies” have been used to bolster claims of effectiveness for Celebrex and other drugs, as the public is fed the fiction that we have the best research in the world and the safest and most effective drugs. Yes, we have the best research results money can buy, and the best “approving for dollars” system too.
This corporate medical/scientific corruption hurts us all, and the media is only too happy to trot out the results of these “studies” while lapping up billions in pharma advertising.
A year earlier, Constantine Kostas admitted that of the 85 subjects in his clinical trial of Cipro, only 15 had actually been given Cipro. I suppose it hardly matters, since Kostas also faked the results of lab tests and examinations that never took place.
All of this is especially irksome to those of us who have endured Big Pharma’s war on herbs and supplements. How many of the so-called “research studies” on herbs funded by big pharma are also bogus? It’s easy to find such “studies” in which the patients chosen were inappropriate, or the protocol designed in ways that appear destined to fail. Indeed, “failure” of an alternative medicine is exactly what the drug companies want to see.
Dangers of Estrogen Replacement Therapy

"Hormone replacement therapy — medications containing female hormones to replace the ones the body no longer makes after menopause — used to be a standard treatment for women with hot flashes and other menopause symptoms. Hormone therapy (as it's now called) was also thought to have the long-term benefits of preventing heart disease and osteoporosis. Attitudes about hormone therapy changed abruptly in 2002, when a large clinical trial found that the treatment actually posed more health risks than benefits for postmenopausal women. As the number of health hazards attributed to hormone therapy grew, doctors became less likely to prescribe it." The Mayo Clinic
It's shocking that few women are told about the dangers involved with taking estrogen hormones. The Information below was taken word for word from a PremarinTM Ad In The June 1998 Issue Of Reader's Digest. Premarin (urine from pregnant mares) is a common source of estrogen.
Cancer of the uterus. The risk of cancer of the uterus increases the longer estrogens are used and when larger doses are taken. One study showed that when estrogens are discontinued, this increased risk of cancer seems to fall off quickly. In another study, the persistence of risk was demonstrated for 10 years after stopping estrogen treatment. Because of this risk, it is important to take the lowest effective dose of estrogen and to take it only as long as you need it. There is a higher risk of cancer of the uterus if you are overweight, diabetic, or have high blood pressure. If you have had your uterus removed (total hysterectomy), there is no danger of developing cancer of the uterus. If you have your uterus, please refer to the section titled "OTHER INFORMATION."
Cancer of the breast. The majority of studies have shown no association with the usual doses for estrogen replacement therapy and breast cancer. Some studies have suggested a possible increased incidence of breast cancer in those women taking estrogens for prolonged periods of time and especially if higher doses are used.
Gallbladder disease. Women who use estrogens after menopause are more likely to develop gallbladder disease needing surgery than women who do not use estrogens.
Abnormal blood clotting. Taking estrogens may increase the risk of blood clots. These clots can cause a stroke, heart attack or pulmonary embolus, any of which may be fatal.
Heart disease. Large doses of estrogen in men have shown to increase the risk of certain heart diseases. This may not necessarily be true in women. In order to avoid the theoretical risk of high doses, the dose of estrogen you take should not exceed the dose recommended by your doctor.
Excess calcium in the blood. Taking estrogens may lead to severe hypercalcemia in women with breast and/or bone cancer.
SIDE EFFECTS
In addition to the risks listed above, the following side effects have been reported with estrogen use:
It's shocking that few women are told about the dangers involved with taking estrogen hormones. The Information below was taken word for word from a PremarinTM Ad In The June 1998 Issue Of Reader's Digest. Premarin (urine from pregnant mares) is a common source of estrogen.
Cancer of the uterus. The risk of cancer of the uterus increases the longer estrogens are used and when larger doses are taken. One study showed that when estrogens are discontinued, this increased risk of cancer seems to fall off quickly. In another study, the persistence of risk was demonstrated for 10 years after stopping estrogen treatment. Because of this risk, it is important to take the lowest effective dose of estrogen and to take it only as long as you need it. There is a higher risk of cancer of the uterus if you are overweight, diabetic, or have high blood pressure. If you have had your uterus removed (total hysterectomy), there is no danger of developing cancer of the uterus. If you have your uterus, please refer to the section titled "OTHER INFORMATION."
Cancer of the breast. The majority of studies have shown no association with the usual doses for estrogen replacement therapy and breast cancer. Some studies have suggested a possible increased incidence of breast cancer in those women taking estrogens for prolonged periods of time and especially if higher doses are used.
Gallbladder disease. Women who use estrogens after menopause are more likely to develop gallbladder disease needing surgery than women who do not use estrogens.
Abnormal blood clotting. Taking estrogens may increase the risk of blood clots. These clots can cause a stroke, heart attack or pulmonary embolus, any of which may be fatal.
Heart disease. Large doses of estrogen in men have shown to increase the risk of certain heart diseases. This may not necessarily be true in women. In order to avoid the theoretical risk of high doses, the dose of estrogen you take should not exceed the dose recommended by your doctor.
Excess calcium in the blood. Taking estrogens may lead to severe hypercalcemia in women with breast and/or bone cancer.
SIDE EFFECTS
In addition to the risks listed above, the following side effects have been reported with estrogen use:
- Nausea, vomiting: pain, cramps, swelling, or tenderness in the abdomen
- Yellowing of the skin and/or whites of the eyes
- Breast tenderness or enlargement
- Enlargement of benign tumors of the uterus
- Breakthrough bleeding or spotting
- Change in amount of cervical secretion
- Vaginal yeast infections
- Retention of excess fluid. This may make some conditions worsen, such as asthma, epilepsy, migraine, heart disease, or kidney disease.
- A spotty darkening of the skin, particularly on the face, reddening of the skin/ skin rashes
- Worsening of porphyria
- Headache, migraines, dizziness, faintness, or changes in vision (including intolerance to contact lenses)
- Mental depression
- Involuntary muscle spasms
- Hair loss or abnormal hairiness
- Increase or decrease in weight
- Changes in sex drive
- Possible changes in blood sugar
Medical Research

"A federally-funded study begun in 1942 injected male patients at a state insane asylum in Ypsilanti, Michigan, with an experimental flu vaccine, then exposed them to flu several months later. It was co-authored by Dr Jonas Salk, who, a decade later, would become famous as inventor of the polio vaccine. Some of the men weren’t able to describe their symptoms, raising serious questions about how well they understood what was being done to them. One newspaper account mentioned the test subjects were “senile and debilitated”.
The noted researcher Dr W Paul Havens Jnr, in federally-funded studies in the 1940s, exposed men to hepatitis; in one he used patients from mental institutions in Middletown and Norwich, Connecticut. Havens, a World Health Organization expert on viral diseases, was one of the first scientists to differentiate types of hepatitis and their causes. The study involving mental patients made eight healthy men ill but broke no new ground in understanding the disease.
Researchers in the mid-1940s studied the transmission of a deadly stomach bug by having young men from New York State Vocational Institution, a prison, swallow unfiltered stool suspension. The point was to see how well the disease spread in comparison with having test subjects breathe germs in. Swallowing was more effective, researchers concluded.
A University of Minnesota study in the late 1940s injected 11 public service employee volunteers with malaria, then starved them for five days. Some were also subjected to hard labour. They were treated with quinine sulphate. One of the study’s authors was Ancel Keys, a dietician who developed K-rations for the military.
For a study in 1957, when the Asian flu pandemic was spreading, federal researchers sprayed the virus in the noses of 23 inmates at Patuxent prison in Jessup, Maryland, to compare their reactions to those of 32 virus-exposed inmates who had been given a new vaccine.
Government researchers in the 1950s tried to infect about two dozen volunteering inmates at a federal penitentiary in Atlanta with gonorrhea. The bacteria was pumped into the urinary tract and the men quickly developed the disease, but it was noted that this method wasn’t comparable with the way men normally became infected, by having sex with an infected partner. The men were later treated with antibiotics.
Though people in the studies were usually described as volunteers, historians and ethicists have questioned how well they understood what was to be done to them and why, or whether they were coerced.
During the Second World War, prisoners were enlisted to help the war effort by taking part in studies that could help the troops. For example, a series of studies at Stateville Penitentiary in Illinois and two other prisons was designed to test antimalarial drugs that could help soldiers fighting in the Pacific.
The prosecution of Nazi doctors in 1947 led to the “Nuremberg Code”, a set of international rules to protect human test subjects. However, many American doctors ignored them, arguing that they applied to Nazi atrocities, not to US medicine.
The late 1940s and 1950s saw huge growth in America’s pharmaceutical and healthcare industries, accompanied by a boom in prisoner experiments funded by both the government and corporations. By the 1960s, at least half the states allowed inmates to be used as medical guinea pigs.
But two studies in the 1960s turned around public attitudes. The first came to light in 1963. Researchers injected cancer cells into 19 old, debilitated patients at Brooklyn’s Jewish Chronic Disease Hospital in New York, to see if their bodies would reject them. The hospital director said the patients were not told they were being injected with cancer cells because there was no need – the cells were deemed harmless. But the experiment upset a lawyer named William Hyman who sat on the hospital’s board of directors. After the state investigated, the hospital declared that any future experiments would require the patient’s written consent.
Then, at nearby Staten Island, from 1963 to 1966, mentally retarded children at Willowbrook State School were given hepatitis, orally and by injection, to see if they could then be cured with gamma globulin.
Those two studies, along with the Tuskegee experiment, which came to light in 1972, sparked extensive critical media coverage and public disgust, said Susan Reverby, the Wellesley College historian who discovered records of the syphilis study in Guatemala.
By the early 1970s, even experiments involving prisoners were considered scandalous. In widely covered congressional hearings in 1973, pharmaceutical industry officials acknowledged they were using prisoners for testing because they were cheaper than chimpanzees. The government responded with reforms. In the mid-1970s, the US Bureau of Prisons in effect excluded all research by drug companies and other outside agencies within federal prisons.
As the supply of prisoners and mental patients dried up, researchers looked to other countries. Clinical trials could be done more cheaply and with fewer rules. And it was easy to find patients who were taking no medication, a factor that can complicate tests of other drugs.
Additional ethical guidelines have been enacted, and few believe that the Guatemala study could happen today. Still, in the past 15 years two international studies have sparked outrage. One was likened to Tuskegee: US-funded doctors failed to give the Aids drug AZT to all HIV-positive pregnant women in a study in Uganda, even though it would have protected their newborns. US health officials said the study would answer questions about AZT’s use in the developing world.
The other study, by Pfizer, gave the antibiotic Trovan to children with meningitis in Nigeria, although there were doubts about its effectiveness. Critics blamed the experiment for the deaths of 11 children and the disabling of scores of others. Pfizer settled a lawsuit with Nigerian officials for $75m but admitted no wrongdoing.
Last year, the inspector-general of the US Department of Health and Human Services reported that in 2008 between 40 and 65 per cent of clinical studies of federally regulated medical products were conducted in other countries; that proportion has probably grown. The report also noted that US regulators inspected fewer than 1 per cent of foreign trial sites". http://healthfreedoms.org/2011/03/14/shameful-past-of-medical-trials-prompts-new-us-investigations/
Rick Perry approved business grants to donors By Steven Nelson - The Daily Caller
...Another grant that raised eyebrows became an election issue during Perry’s successful campaign for a third term in 2010. In August of that year, Convergen LifeSciences Inc. received a $4.5 million grant from the fund. That company focuses on developing cancer therapies.
The Convergen grant circumvented a regional approval board, a highly unusual move, after it failed to win its approval. Convergen owner David Nance donated approximately $80,000 to Perry’s political campaigns between 2000 and 2010, according to figures made public by the Texas Ethics Commission.
Perry’s Democratic opponent in the gubernatorial race, former Houston Mayor Bill White, used this news as an occasion to allege a “pattern of corruption” in Perry’s political history, proclaiming that “for those companies that open their pockets with campaign contributions, indeed the governor’s office has been open for business.”
“Nance’s application for the money did not follow usual channels for approval,” The Austin American-Statesman reported. ”An Austin-area screening board rejected the initial application, and then Nance sidestepped another screening by a board that focuses on life sciences applications.”
“Instead,” the paper reported, “he took his application to a 17-member statewide advisory board, made up mostly of Perry appointees, and asked Alan Kirchhoff, Perry’s director of economic development at the time, to intervene.”
Perry trounced White in the gubernatorial election. Three days later, the governor released the contract with Convergen, “after first contending it should be kept secret,” the American-Statesman reported.
“Here’s a successful Texas investment strategy,” Ladd wrote. “Invest $75,000 in the Governor’s campaigns. Then fund the rest of your business with a $4.5m taxpayer funded grant from the Governor’s ETF. That’s not a loan like Solyndra received, that’s cash on the barrelhead, delivered from the state and never to be repaid.”
Minnesota Rep. Michele Bachmann has taken aim at an award issued by a similar, but distinct, fund in Texas: the Texas Enterprise Fund. Created in 2003, that program has doled out more than $400 million to companies.
Bachmann targeted a $35 million grant to Lexicon Pharmaceuticals Inc, alleging that Perry “gave $35 million and a grant to a private company and there were donors in that private company.” Bachmann noted that Lexicon failed to meet its promise of creating jobs, actually reducing its workforce.
Other issues involving these investment funds have caught the attention of the Texas press. The Dallas Morning News reported in 2010 that Verve Public Relations Inc., a company operated by Christiane “CJ” Nance, was paid $70,000 to produce promotional videos supportive of the Emerging Technology Fund. CJ Nance’s father is Convergen owner David Nance. He supplied $100,000 for the promotional work.
IRS adviser James P. Joseph told the Morning News that the situation was “highly unusual” and “looks very suspicious.” A donation in the form of an “earmarked grant to pay your child, that’s the definition of an insider transaction,” Joseph said, suggesting that the IRS might be interested in whether Nance reported the donation on tax returns.
According to the Morning News, grants totaling $16 million awarded by the Emerging Technology Fund have gone to major Perry donors.
The Club for Growth, a fiscal conservative advocacy group, has expressed concern that the two development funds “create huge market distortions in a place that should naturally be a nationwide leader in attracting jobs.” The group cited Perry’s role in the funds a evidence that “he has at times an interventionist streak rather than consistent free-market principles.”
The noted researcher Dr W Paul Havens Jnr, in federally-funded studies in the 1940s, exposed men to hepatitis; in one he used patients from mental institutions in Middletown and Norwich, Connecticut. Havens, a World Health Organization expert on viral diseases, was one of the first scientists to differentiate types of hepatitis and their causes. The study involving mental patients made eight healthy men ill but broke no new ground in understanding the disease.
Researchers in the mid-1940s studied the transmission of a deadly stomach bug by having young men from New York State Vocational Institution, a prison, swallow unfiltered stool suspension. The point was to see how well the disease spread in comparison with having test subjects breathe germs in. Swallowing was more effective, researchers concluded.
A University of Minnesota study in the late 1940s injected 11 public service employee volunteers with malaria, then starved them for five days. Some were also subjected to hard labour. They were treated with quinine sulphate. One of the study’s authors was Ancel Keys, a dietician who developed K-rations for the military.
For a study in 1957, when the Asian flu pandemic was spreading, federal researchers sprayed the virus in the noses of 23 inmates at Patuxent prison in Jessup, Maryland, to compare their reactions to those of 32 virus-exposed inmates who had been given a new vaccine.
Government researchers in the 1950s tried to infect about two dozen volunteering inmates at a federal penitentiary in Atlanta with gonorrhea. The bacteria was pumped into the urinary tract and the men quickly developed the disease, but it was noted that this method wasn’t comparable with the way men normally became infected, by having sex with an infected partner. The men were later treated with antibiotics.
Though people in the studies were usually described as volunteers, historians and ethicists have questioned how well they understood what was to be done to them and why, or whether they were coerced.
During the Second World War, prisoners were enlisted to help the war effort by taking part in studies that could help the troops. For example, a series of studies at Stateville Penitentiary in Illinois and two other prisons was designed to test antimalarial drugs that could help soldiers fighting in the Pacific.
The prosecution of Nazi doctors in 1947 led to the “Nuremberg Code”, a set of international rules to protect human test subjects. However, many American doctors ignored them, arguing that they applied to Nazi atrocities, not to US medicine.
The late 1940s and 1950s saw huge growth in America’s pharmaceutical and healthcare industries, accompanied by a boom in prisoner experiments funded by both the government and corporations. By the 1960s, at least half the states allowed inmates to be used as medical guinea pigs.
But two studies in the 1960s turned around public attitudes. The first came to light in 1963. Researchers injected cancer cells into 19 old, debilitated patients at Brooklyn’s Jewish Chronic Disease Hospital in New York, to see if their bodies would reject them. The hospital director said the patients were not told they were being injected with cancer cells because there was no need – the cells were deemed harmless. But the experiment upset a lawyer named William Hyman who sat on the hospital’s board of directors. After the state investigated, the hospital declared that any future experiments would require the patient’s written consent.
Then, at nearby Staten Island, from 1963 to 1966, mentally retarded children at Willowbrook State School were given hepatitis, orally and by injection, to see if they could then be cured with gamma globulin.
Those two studies, along with the Tuskegee experiment, which came to light in 1972, sparked extensive critical media coverage and public disgust, said Susan Reverby, the Wellesley College historian who discovered records of the syphilis study in Guatemala.
By the early 1970s, even experiments involving prisoners were considered scandalous. In widely covered congressional hearings in 1973, pharmaceutical industry officials acknowledged they were using prisoners for testing because they were cheaper than chimpanzees. The government responded with reforms. In the mid-1970s, the US Bureau of Prisons in effect excluded all research by drug companies and other outside agencies within federal prisons.
As the supply of prisoners and mental patients dried up, researchers looked to other countries. Clinical trials could be done more cheaply and with fewer rules. And it was easy to find patients who were taking no medication, a factor that can complicate tests of other drugs.
Additional ethical guidelines have been enacted, and few believe that the Guatemala study could happen today. Still, in the past 15 years two international studies have sparked outrage. One was likened to Tuskegee: US-funded doctors failed to give the Aids drug AZT to all HIV-positive pregnant women in a study in Uganda, even though it would have protected their newborns. US health officials said the study would answer questions about AZT’s use in the developing world.
The other study, by Pfizer, gave the antibiotic Trovan to children with meningitis in Nigeria, although there were doubts about its effectiveness. Critics blamed the experiment for the deaths of 11 children and the disabling of scores of others. Pfizer settled a lawsuit with Nigerian officials for $75m but admitted no wrongdoing.
Last year, the inspector-general of the US Department of Health and Human Services reported that in 2008 between 40 and 65 per cent of clinical studies of federally regulated medical products were conducted in other countries; that proportion has probably grown. The report also noted that US regulators inspected fewer than 1 per cent of foreign trial sites". http://healthfreedoms.org/2011/03/14/shameful-past-of-medical-trials-prompts-new-us-investigations/
Rick Perry approved business grants to donors By Steven Nelson - The Daily Caller
...Another grant that raised eyebrows became an election issue during Perry’s successful campaign for a third term in 2010. In August of that year, Convergen LifeSciences Inc. received a $4.5 million grant from the fund. That company focuses on developing cancer therapies.
The Convergen grant circumvented a regional approval board, a highly unusual move, after it failed to win its approval. Convergen owner David Nance donated approximately $80,000 to Perry’s political campaigns between 2000 and 2010, according to figures made public by the Texas Ethics Commission.
Perry’s Democratic opponent in the gubernatorial race, former Houston Mayor Bill White, used this news as an occasion to allege a “pattern of corruption” in Perry’s political history, proclaiming that “for those companies that open their pockets with campaign contributions, indeed the governor’s office has been open for business.”
“Nance’s application for the money did not follow usual channels for approval,” The Austin American-Statesman reported. ”An Austin-area screening board rejected the initial application, and then Nance sidestepped another screening by a board that focuses on life sciences applications.”
“Instead,” the paper reported, “he took his application to a 17-member statewide advisory board, made up mostly of Perry appointees, and asked Alan Kirchhoff, Perry’s director of economic development at the time, to intervene.”
Perry trounced White in the gubernatorial election. Three days later, the governor released the contract with Convergen, “after first contending it should be kept secret,” the American-Statesman reported.
“Here’s a successful Texas investment strategy,” Ladd wrote. “Invest $75,000 in the Governor’s campaigns. Then fund the rest of your business with a $4.5m taxpayer funded grant from the Governor’s ETF. That’s not a loan like Solyndra received, that’s cash on the barrelhead, delivered from the state and never to be repaid.”
Minnesota Rep. Michele Bachmann has taken aim at an award issued by a similar, but distinct, fund in Texas: the Texas Enterprise Fund. Created in 2003, that program has doled out more than $400 million to companies.
Bachmann targeted a $35 million grant to Lexicon Pharmaceuticals Inc, alleging that Perry “gave $35 million and a grant to a private company and there were donors in that private company.” Bachmann noted that Lexicon failed to meet its promise of creating jobs, actually reducing its workforce.
Other issues involving these investment funds have caught the attention of the Texas press. The Dallas Morning News reported in 2010 that Verve Public Relations Inc., a company operated by Christiane “CJ” Nance, was paid $70,000 to produce promotional videos supportive of the Emerging Technology Fund. CJ Nance’s father is Convergen owner David Nance. He supplied $100,000 for the promotional work.
IRS adviser James P. Joseph told the Morning News that the situation was “highly unusual” and “looks very suspicious.” A donation in the form of an “earmarked grant to pay your child, that’s the definition of an insider transaction,” Joseph said, suggesting that the IRS might be interested in whether Nance reported the donation on tax returns.
According to the Morning News, grants totaling $16 million awarded by the Emerging Technology Fund have gone to major Perry donors.
The Club for Growth, a fiscal conservative advocacy group, has expressed concern that the two development funds “create huge market distortions in a place that should naturally be a nationwide leader in attracting jobs.” The group cited Perry’s role in the funds a evidence that “he has at times an interventionist streak rather than consistent free-market principles.”
Heartburn Drugs and Hip Fractures
(from http://www.peoplespharmacy.com/2012/02/01/heartburn-drugs-and-hip-fractures/ )
Suppressing acid formation in the stomach with a class of medications called proton pump inhibitors (PPIs) seemed like a great plan. Such drugs have become incredibly popular:
• Esomeprazole (Nexium)
• Lansoprazole (Prevacid)
• Omeprazole (Prilosec)
• Pantoprazole (Protonix)
• Rabeprazole (Aciphex)
You can now buy omeprazole (Prilosec) and lansoprazole (Prevacid) over the counter without medical supervision. Not surprisingly, these drugs are among the most popular pills for heartburn.
But there are a couple of problems. There is acid in the stomach for a reason. It was not a mistake of nature that most animals have highly concentrated acid in their stomachs. Acid is necessary to digest food and allow for absorption of certain key nutrients. Acid also kills lots of nasty germs that might get into our stomachs from food, water and other sources. Suppressing acid so effectively may increase our risk for certain infections. It also seems to increase the risk for a very serious and unanticipated complication: hip fracture!
An article just out from the BMJ (once known as the British Medical Journal) reveals that postmenopausal women who smoked (current or former smokers) and took a proton pump inhibitor for at least two years were at greater rid for hip fractures. Nearly 80,000 women from the Nurses' Health Study were followed for roughly eight years. That represented over 500,000 person years of follow-up. There were about 900 hip fractures in this group of women. Compared with with women who never used PPIs the women who regularly relied on these acid suppressing drugs had a 35 percent greater risk of hip fracture. Smokers had a 50 percent increased risk for hip fracture.
In pharmacology we like to look at dose response curves and length of exposure to a drug to determine risk. In this case, the longer these women swallowed a powerful acid suppressing drug, the stronger the likelihood that they would experience a fracture.
This is not the first time research has suggested a link between acid suppressing drugs and hip fracture. When all the data are analyzed together the risk of hip fracture is increased by about 30 percent. Although the mechanism has not yet been nailed down, three possibilities have been considered. One, these drugs may interfere with calcium absorption, necessary for proper bone formation. Two, PPIs may directly interfere with the cells that help remodel bone and keep it strong. Three, by inhibiting acid formation, such medications increase the body's production of gastrin, necessary for food digestion. Too much gastrin could negatively impact bone mineral density.
Based on the new finding from the Nurses' Health Study and prior research, we now think that there is reason to be concerned about PPIs and and a link to hip fractures. The difficulty appears to disappear once such drugs have been discontinued for at least two years.
There is a problem, however. Stopping PPIs after several months of regular use can be difficult. Rebound hyperacidity (really bad heartburn) is a common complaint. You can read about strategies to get off PPIs by checking out this link and this link.
Of course we would suggest that anyone who considers stopping a PPI check with the prescribing physician first. Some people may have to stay on these drugs indefinitely because of Barrett's esophagus.
Suppressing acid formation in the stomach with a class of medications called proton pump inhibitors (PPIs) seemed like a great plan. Such drugs have become incredibly popular:
• Esomeprazole (Nexium)
• Lansoprazole (Prevacid)
• Omeprazole (Prilosec)
• Pantoprazole (Protonix)
• Rabeprazole (Aciphex)
You can now buy omeprazole (Prilosec) and lansoprazole (Prevacid) over the counter without medical supervision. Not surprisingly, these drugs are among the most popular pills for heartburn.
But there are a couple of problems. There is acid in the stomach for a reason. It was not a mistake of nature that most animals have highly concentrated acid in their stomachs. Acid is necessary to digest food and allow for absorption of certain key nutrients. Acid also kills lots of nasty germs that might get into our stomachs from food, water and other sources. Suppressing acid so effectively may increase our risk for certain infections. It also seems to increase the risk for a very serious and unanticipated complication: hip fracture!
An article just out from the BMJ (once known as the British Medical Journal) reveals that postmenopausal women who smoked (current or former smokers) and took a proton pump inhibitor for at least two years were at greater rid for hip fractures. Nearly 80,000 women from the Nurses' Health Study were followed for roughly eight years. That represented over 500,000 person years of follow-up. There were about 900 hip fractures in this group of women. Compared with with women who never used PPIs the women who regularly relied on these acid suppressing drugs had a 35 percent greater risk of hip fracture. Smokers had a 50 percent increased risk for hip fracture.
In pharmacology we like to look at dose response curves and length of exposure to a drug to determine risk. In this case, the longer these women swallowed a powerful acid suppressing drug, the stronger the likelihood that they would experience a fracture.
This is not the first time research has suggested a link between acid suppressing drugs and hip fracture. When all the data are analyzed together the risk of hip fracture is increased by about 30 percent. Although the mechanism has not yet been nailed down, three possibilities have been considered. One, these drugs may interfere with calcium absorption, necessary for proper bone formation. Two, PPIs may directly interfere with the cells that help remodel bone and keep it strong. Three, by inhibiting acid formation, such medications increase the body's production of gastrin, necessary for food digestion. Too much gastrin could negatively impact bone mineral density.
Based on the new finding from the Nurses' Health Study and prior research, we now think that there is reason to be concerned about PPIs and and a link to hip fractures. The difficulty appears to disappear once such drugs have been discontinued for at least two years.
There is a problem, however. Stopping PPIs after several months of regular use can be difficult. Rebound hyperacidity (really bad heartburn) is a common complaint. You can read about strategies to get off PPIs by checking out this link and this link.
Of course we would suggest that anyone who considers stopping a PPI check with the prescribing physician first. Some people may have to stay on these drugs indefinitely because of Barrett's esophagus.
The FDA

Consumer Reports has identified 12 relatively common prescription-drug types linked to serious risks--including an increased likelihood of heart attack, stroke, cancer, or suicide--that were undetected or underestimated when they were approved for use. Many of those drugs are still being advertised, and some lack a “black box” warning that our chief medical adviser says is needed. Indeed, the nation's drug-safety system has extensive weaknesses that put users of prescription medicines at risk:
•Rush to approve. Working under tight deadlines and with sometimes skimpy, unrepresentative data, the Food and Drug Administration reviews drugs at a pace that may make sound decisions difficult. Some FDA scientists say that their bosses have pushed them to approve medications despite their reservations about safety.
•A powerless FDA. The agency lacks the power to compel companies to complete studies after drug approval, force doctors to report adverse reactions, or dictate new warning labels. Its Office of Drug Safety assesses risks that emerge after approval, but it's understaffed and is not permitted to make the final decision about those risks.
•Risks hidden. Some companies have withheld studies showing unexpected risks or poor efficacy, which might limit use of a drug. And our analysis of FDA letters to drug firms indicates a broad spectrum of misleading promotions, which have continued unabated through September 2005; such ads swell demand for new drugs before their risks are fully known.
•Reforms needed. Despite recent signs of improvement at the FDA, extensive reforms are needed in the drug-approval process, the monitoring of risks that emerge after approval, and the regulation of drug ads.
Bent Penis Syndrome
Peyronie's disease involves a patch of tissue in the penis that becomes fibrous and does not expand normally as the rest of the tissue does. This "plaque" is responsible for the bend in the erection.
Because trauma is believed to be a contributing factor in some cases, men should be warned that attempting intercourse with an incomplete erection could increase their risk. Sexual acrobatics that put a strain on the erect penis may also lead to tears or bruises.
Some medications may place a man at higher risk of developing this disease. Beta blockers for heart and blood pressure treatment such as Blocadren, Cartrol, Inderal, Lopressor, Normodyne, Tenormin, Toprol XL, Trandate and Visken, glaucoma eye drops such as Timoptic, the migraine tablet Sansert and the seizure medicine Dilantin have all been associated with Peyronie's. The injected impotence drug Caverject (alprostadil) may also cause fibrous tissue build-up. Careful monitoring by a urologist is essential. http://www.peoplespharmacy.com/2011/07/06/sexual-acrobatics-kink-penis/
FDA Pregnancy Classifications
The FDA is lacking a great deal of information about most medicines and the effects they have on unborn children. To help women make informed decisions about medications, the FDA assigns categories to medications. The safest drugs fall under category A, where there is no known risk to the fetus during the pregnancy. The FDA classifications are as follows:
A – No studies have shown any risk to the fetus during pregnancy.
B – No adequate studies exist to rule out danger to the fetus. The drug may provide beefits that overrule the potential dangers.
C – Studies in animals show dangers to the fetus, but no adequate human studies exist. The drug may provide benefits that overrule the potential dangers.
D – Studies have clearly demonstrated risk to the human fetus based on real world experiences or human studies. The drug may provide benefits that overrule the potential dangers.
X – Studies clearly show a risk of fetal abnormalities in animals or humans and the potential benefits of the drug are not enough to overcome the dangers.
While these classifications protect women from known dangers, they can do little else. Another problem with the classification system lies in the vague wording that leaves them open to wide interpretation. Until a drug’s dangers become known, women and their children remain at risk.
FDA Admits Classifications are a Problem
Karen Feibus works as a medical team leader at the FDA’s office of new drugs. She admits the problem, saying, “You may have drugs with no data on reproductive toxicity at all, and drugs where a few animal offspring showed some malformations, and drugs where studies showed the frequent major malformations in three different species, and they’re all lumped together in category C.”[2]
Testing Information Complicated
To make matters worse, the literature that comes with medications describes the manufacturer’s clinical trials in scientific jargon, making them hard for many women to understand. This leaves women at the mercy of their doctors’ interpretations, instead of giving them access to easily understandable information.
Labeling Changes May Make Matters Worse
The FDA is moving away from pregnancy categories in an effort to provide clearer information, but the new rules may instead muddy the waters more. Drug labels will include summaries of known pregnancy risks with considerations doctors should take into account. This may be confusing for patients, leading pregnant women to assume a drug is safe because no human studies show a risk to the fetus. http://www.cssfirm.com/2011/05/13/fda-drug-classifications-fail-to-protect-pregnant-women/
•Rush to approve. Working under tight deadlines and with sometimes skimpy, unrepresentative data, the Food and Drug Administration reviews drugs at a pace that may make sound decisions difficult. Some FDA scientists say that their bosses have pushed them to approve medications despite their reservations about safety.
•A powerless FDA. The agency lacks the power to compel companies to complete studies after drug approval, force doctors to report adverse reactions, or dictate new warning labels. Its Office of Drug Safety assesses risks that emerge after approval, but it's understaffed and is not permitted to make the final decision about those risks.
•Risks hidden. Some companies have withheld studies showing unexpected risks or poor efficacy, which might limit use of a drug. And our analysis of FDA letters to drug firms indicates a broad spectrum of misleading promotions, which have continued unabated through September 2005; such ads swell demand for new drugs before their risks are fully known.
•Reforms needed. Despite recent signs of improvement at the FDA, extensive reforms are needed in the drug-approval process, the monitoring of risks that emerge after approval, and the regulation of drug ads.
Bent Penis Syndrome
Peyronie's disease involves a patch of tissue in the penis that becomes fibrous and does not expand normally as the rest of the tissue does. This "plaque" is responsible for the bend in the erection.
Because trauma is believed to be a contributing factor in some cases, men should be warned that attempting intercourse with an incomplete erection could increase their risk. Sexual acrobatics that put a strain on the erect penis may also lead to tears or bruises.
Some medications may place a man at higher risk of developing this disease. Beta blockers for heart and blood pressure treatment such as Blocadren, Cartrol, Inderal, Lopressor, Normodyne, Tenormin, Toprol XL, Trandate and Visken, glaucoma eye drops such as Timoptic, the migraine tablet Sansert and the seizure medicine Dilantin have all been associated with Peyronie's. The injected impotence drug Caverject (alprostadil) may also cause fibrous tissue build-up. Careful monitoring by a urologist is essential. http://www.peoplespharmacy.com/2011/07/06/sexual-acrobatics-kink-penis/
FDA Pregnancy Classifications
The FDA is lacking a great deal of information about most medicines and the effects they have on unborn children. To help women make informed decisions about medications, the FDA assigns categories to medications. The safest drugs fall under category A, where there is no known risk to the fetus during the pregnancy. The FDA classifications are as follows:
A – No studies have shown any risk to the fetus during pregnancy.
B – No adequate studies exist to rule out danger to the fetus. The drug may provide beefits that overrule the potential dangers.
C – Studies in animals show dangers to the fetus, but no adequate human studies exist. The drug may provide benefits that overrule the potential dangers.
D – Studies have clearly demonstrated risk to the human fetus based on real world experiences or human studies. The drug may provide benefits that overrule the potential dangers.
X – Studies clearly show a risk of fetal abnormalities in animals or humans and the potential benefits of the drug are not enough to overcome the dangers.
While these classifications protect women from known dangers, they can do little else. Another problem with the classification system lies in the vague wording that leaves them open to wide interpretation. Until a drug’s dangers become known, women and their children remain at risk.
FDA Admits Classifications are a Problem
Karen Feibus works as a medical team leader at the FDA’s office of new drugs. She admits the problem, saying, “You may have drugs with no data on reproductive toxicity at all, and drugs where a few animal offspring showed some malformations, and drugs where studies showed the frequent major malformations in three different species, and they’re all lumped together in category C.”[2]
Testing Information Complicated
To make matters worse, the literature that comes with medications describes the manufacturer’s clinical trials in scientific jargon, making them hard for many women to understand. This leaves women at the mercy of their doctors’ interpretations, instead of giving them access to easily understandable information.
Labeling Changes May Make Matters Worse
The FDA is moving away from pregnancy categories in an effort to provide clearer information, but the new rules may instead muddy the waters more. Drug labels will include summaries of known pregnancy risks with considerations doctors should take into account. This may be confusing for patients, leading pregnant women to assume a drug is safe because no human studies show a risk to the fetus. http://www.cssfirm.com/2011/05/13/fda-drug-classifications-fail-to-protect-pregnant-women/
MRI, MRA or CT Scan
Gadolinium is linked to a New Man Made Disease with No Known Treatment
Posted by Armand RossettiSeptember 17, 2008 http://westpalmbeach.injuryboard.com/medical-devices-and-implants/gadolinium-is-linked-to-a-new-disease-with-no-known-treatment.aspx?googleid=247660
The relatively new medical device is gadolinium-based contrast agent (GBCA or gadolinium) used during certain MRI or similar procedures. The new kidney related disease that is little more than a decade old is called Nephrogenic Systemic Fibrosis (NSF). Caretakers inject the GBCA into the bloodstream just before patients undergo certain types of Magnetic Resonance Imaging (MRI).
Here is an example of how NSF might occur: Patients undergo an MRI, MRA, or CT scan. As part of the procedure, doctors inject the patient with a contrast solution containing a rare earth metal called gadolinium. There are different commercial names for gadolinium-based contrast marker: Omniscan (General Electric), Magnavist (Bayer Healthcare), OptiMark (Mallinckrodt), MultiHance (Bracco), and ProHance (Bracco).
After being injected, the Gadolinium dissipates throughout the body. Ordinarily, gadolinium is extremely toxic to human tissues, and manufacturers have to chelate or coat gadolinium with benign chemicals to make it safe for use as an internal marker. Once the chelated gadolinium disperses in the body, doctors can use MRI to obtain a sharper image than they might have obtained without using gadolinium.
Once the MRI procedure is complete, there is no longer a need for chelated gadolinium to be circulating in the bloodstream or to be bound to other body tissue, and the kidneys usually remove the coated gadolinium from the blood. However, patients who have poor kidney function have less capacity to remove gadolinium from the body. This incapacity to remove gadolinium at a preferred rate leads to persistently high levels of residual gadolinium in body tissue. Compromised kidneys’ lack the “speed” necessary to remove gadolinium, and this failure to eliminate the gadolinium leads to a more dangerous situation.
The chelated gadolinium that remains behind in the bodies of patients with poor kidney function then goes through a further process where the protective chelate breaks off of the gadolinium, and free, very toxic, gadolinium remains. Lack of kidney function permits greater amounts of free gadolinium to circulate in the body. And gadolinium toxicity then begins to manifest in vital organs located outside the circulatory system.
For example, after free gadolinium reaches the skin, it forms deposits that cause the skin to become less elastic, patchy and discolored. Skin changes include blackening of tissue and scarring, which deforms and hardens the skin. The hardening and deformation of the skin leads to substantial pain and loss of flexibility while moving the arms and walking. Muscle tissue also absorbs the toxic gadolinium, causing further pain and weakness, with notable hip involvement.
Free gadolinium also deposits in the eyes, and in vital organs, such as the lungs, heart, and liver. Gadolinium deposits in vital organs often result in fatal organ failure.
Once again symptoms of gadolinium poisoning include:
Hardening and darkening of the skin;
Dark patches on the skin;
Burning sensations;
Painful joints and stiffness;
Difficulty straightening the arms, legs and feet;
Weakness;
Yellow patches on the eyes; and
Hip pain.
Next we might ask why gadolinium escaped detection as a potential hazard.
Thyroid Problems by Dr. John Lowe
"I learned early during the last 16 years that the endocrinology specialty's judgment is corrupted by financial inducements from drug companies that profit from the TSH test and T4 replacement. All those years ago, my intention was to help revise the often harmful standard of care imposed by the endocrinology specialty for commercial rather than scientific reasons.
After careful consideration in the last several years, however, I realized that the specialty has its heels dug in; it's clear to me that the specialty won't volitionally rehabilitate itself into a rational, scientific, ethical, and respectable medical specialty.
Rather than rehabilitate, to this day, the specialty practices thuggery on a par with that of traditional organized crime. It does so by intimidating and persecuting clinicians who fail to cooperate in restricting their patients to T4 replacement—an often ineffective and harmful approach to therapy that's hugely profitable to Big Pharma and, by quid pro quo, to the specialty itself.
The well-intending persecuted clinicians are guilty of recognizing that T4 replacement doesn't work for and harms many patients. And they are guilty of having the courage to abide by the Hippocratic oath in using thyroid hormone therapies that get their patients well.
The thuggery of the specialty is in highly active gear. We know this because regularly, clinicians contact us and tell us of actions being taken against them by medical regulatory boards. Invariably, the action involves testimony against the clinicians by members of the endocrinology specialty or affiliates of theirs.
And what is the ultimate consequence of these actions against so many well-intended clinicians? Patients who need safe and effective thyroid hormone therapy are restricted to T4 replacement—an approach that published studies clearly show to be ineffective and harmful for many patients.
It seems that most every community has members of the endocrinology specialty who function as thugs. They act as enforcers of the command that clinicians use only TSH testing and T4 products. These thug endocrinologists file complaints against noncompliant clinicians. Then they testify against the clinicians before regulatory boards and courts and walk away scot-free after giving scientifically-false testimony in courts of law and other legal venues.
Experience tells me that the endocrinology specialty won't relent—not until it's forced to do so, probably by class action law suits and by forcible complaints for violations of medical ethics to purveyors of medical regulatory boards. To me, the final solution lies in the education of patients, clinicians, and legislators; legislation to disempower the endocrinology specialty from further harm to patients, clinicians, and the public welfare; and litigation against the specialty and its corporation supporters. The force of unforgiving corruption by the specialty and its affiliates has long loomed over me and my medical colleagues. .. All best wishes, Dr. Lowe."
Nuclear Power Threats Wednesday, August 24, 2011 :: Staff infoZine
Washington D.C. - infoZine -Robert Alvarez ([email protected] ) is a former senior policy adviser to the U.S. Secretary of Energy and now a senior scholar at the Institute for Policy Studies. In June, Alvarez wrote a piece titled "America's Nuclear Spent-Fuel Time Bombs: Japan's nuclear disaster should serve as a wake-up call for the United States." link
He said earlier this afternoon: "The earthquake measuring 5.9 on the Richter scale just occurred less than a hour ago. Its epicenter was in Mineral, Virginia -- approximately ten miles from two nuclear power reactors at the North Anna site. According to statement by a representative of Dominion Power the two reactors were designed to withstand a 5.9 to 6.1 quake. The Nuclear Regulatory Commission ranked the North Anna Reactors as being 7th in the nation in terms of earthquake risks.
"It is not clear what damage might have been sustained at the nuclear site. The North Anna reactors are of the Westinghouse Pressurized Water design and went on line in 1979 and 1980 respectively. Since then the reactors have generated approximately 1,200 metric tons of nuclear spent fuel containing about 228,000 curies of highly radioactive materials -- among the largest concentrations of radioactivity in the United States.
"The spent fuel pools at North Anna contain four to five times more than their original designs intended. As in Japan, all U.S. power nuclear power plant spent fuel pools do not have steel-lined, concrete barriers that cover reactor vessels to prevent the escape of radioactivity. They are not required to have back-up generators to keep used fuel rods cool, if offsite power is lost. Even though they contain some of the largest concentrations of radioactivity on the planet, spent reactor fuel pools in the U.S. are mostly contained in ordinary industrial structures designed to protect them against the elements. The North Anna reactors may have large cavities beneath their pools which could exacerbate leakage.
"Nearly 40 percent of the radioactivity in the North Anna spent fuel pools is cesium-137. They hold about 15 to 30 times more Cs-137 than was released by the Chernobyl accident in 1986. In 2003, my colleagues and I issued a study that warned that drainage of a pool might cause a catastrophic radiation fire, which could render an area uninhabitable greater than that created by the Chernobyl accident. A year later the National Academy of Sciences confirmed our findings and warned that “...partially or completely a spent fuel pool could lead to a propagating zirconium cladding fire and release large quantities of radioactive materials to the environment. ... Such fires would create thermal plumes that could potentially transport radioactive aerosols hundreds of miles downwind under appropriate atmospheric conditions.
Drug Firms Told to Pay $162M in Hepatitis C Case 10-10-2011” LAS VEGAS (AP)
— A Nevada jury on Monday ordered three pharmaceutical companies to pay $162.5 million in punitive damages in a lawsuit that accused them of negligently distributing large vials of an anesthetic to Las Vegas clinics at the center of a 2008 hepatitis C outbreak.
The damages awarded in Clark County District Court are on top of the $20.1 million in compensatory damages awarded to five plaintiffs Thursday after a jury found Teva Parenteral Medicines Inc., Baxter Healthcare Corp. and McKesson Corp. liable.
Plaintiffs' lawyers had accused the companies of putting corporate profits ahead of patient safety, and of recklessly distributing 50 milliliter vials of the powerful anesthetic Propofol to clinics where 10 or 20 milliliter doses were commonly needed for outpatient colonoscopy procedures. They had sought $600 million in punitive damages.
Teva attorney Mark Tully declined comment to reporters after the verdict was read, while photographers and news cameras snapped pictures of family members of the plaintiffs hugging each other and their lawyers.
Philip Hymanson, attorney for Baxter and McKesson, told jurors the Propofol was manufactured properly and delivered properly, and that clinic doctors and anesthesiologists were at fault if they misused it. Hymanson said there was no proof that happened. The companies maintain the vials were properly marked with instructions and warnings, and that jurors weren't allowed to hear that reusing syringes on multiple patients and not following proper sterilizing procedures could also have spread the incurable liver disease.
Teva was ordered to pay $89.4 million, while Baxter was told to pay $55.3 million and McKesson was ordered to pay $17.9 million.
The civil trials are the first of several now reaching trial phases in Las Vegas stemming from the 2008 hepatitis outbreak traced to colonoscopy clinics run by Dr. Dipak Desai. Southern Nevada health officials advised about 50,000 patients who received endoscopy procedures at Desai clinics to be tested for hepatitis. At least nine and as many as 114 patients were infected with the disease.
Another Clark County District Court jury last year found Teva and Baxter liable for damages in a similar case and awarded a combined $500 million in punitive damages to a Las Vegas private school principal and his wife. The man, Henry Chanin, claimed he contracted hepatitis C during a routine endoscopy procedure in 2006. Oskar Garcia can be reached on Twitter at http://twitter.com/oskargarcia
FDA Revokes Approval of Avastin for Breast Cancer WASHINGTON By LAURAN NEERGAARD
...the Food and Drug Administration said it appeared to be a false hope for breast cancer: Studies haven't found that it helps those patients live longer or brings enough other benefit to outweigh its dangerous side effects. "I did not come to this decision lightly," said the FDA's commissioner, Dr. Margaret Hamburg. But she said, "Sometimes despite the hopes of investigators, patients, industry and even the FDA itself, the results of rigorous testing can be disappointing."
Avastin remains on the market to treat certain colon, lung, kidney and brain cancers. Doctors are free to prescribe any marketed drug as they see fit. So even though the FDA formally revoked Avastin's approval as a breast cancer treatment, women could still receive it -- but their insurers may not pay for it. Some insurers already have quit in anticipation of FDA's long-expected ruling.
However, "Medicare will continue to cover Avastin," said Brian Cook, spokesman for the Centers for Medicare & Medicaid Services. The agency "will monitor the issue and evaluate coverage options as a result of action by the FDA but has no immediate plans to change coverage policies." Including infusion fees, a year's treatment with Avastin can reach $100,000....
The Avastin saga began in 2008, when an initial study suggested the drug could delay tumor growth for a few months in women whose breast cancer had spread to other parts of the body. Over the objection of its own advisers and to the surprise of cancer groups, FDA gave Avastin conditional approval -- it could be sold for such women while manufacturer Genentech tried to prove it really worked.
The problem: Ultimately, the tumor effect was even smaller than first thought. Across repeated studies, Avastin patients didn't live longer or have a higher quality of life. Yet the drug causes some life-threatening risks, including severe high blood pressure, massive bleeding, heart attack or heart failure and tears in the stomach and intestines, the FDA concluded. In two public hearings -- one last year and one this summer -- FDA advisers urged the agency to revoke that approval.
"The science is clear: Breast cancer patients are more likely to be harmed than helped by Avastin," said Diana Zuckerman of the National Research Center for Women and Families in Washington. Genentech had argued the drug should remain available while it conducted more research to see if certain subsets of breast cancer patients might benefit, and some patients and their doctors had argued passionately for the drug.
Petrochemicals in Our Lives from http://www.nutramed.com/environment/carsepa.htm
The Combustion Process Gasoline and diesel fuels are mixtures of hydrocarbons (made of hydrogen, oxygen and carbon atoms.) Hydrocarbons are burned by combining with oxygen. Nitrogen and sulphur atoms are also present and combine with oxygen when burned to produce gases. Automotive engines emit several types of pollutants.
Typical Engine Combustion:
Fuel + Air => Hydrocarbons + Nitrogen Oxides + Carbon Dioxide + Carbon Monoxide + water
Hydrocarbon emissions are fragments of fuel molecules, only partially burned. Hydrocarbons react in the presence of nitrogen oxides and sunlight to form ground-level ozone, a major component of smog. Ozone irritates the eyes, nose, throat and damages the lungs. A number of exhaust hydrocarbons are also toxic, some with the potential to cause cancer.
Nitrogen Oxides Under high pressure and temperature conditions in an engine, nitrogen and oxygen atoms react to form nitrogen oxides. Catalytic converters in car exhaust systems break down heavier nitrogen gases, forming nitrogen dioxide (NO2) - 300 times more potent than carbon dioxide as a greenhouse gas. NO2 makes up about 7.2 percent of the gases that cause global warming. Vehicles with catalytic converters produced nearly half of that NO2. NO2 also originates from nitrogen-based fertilizers and manure from farm animals.
Carbon Monoxide Carbon monoxide (CO) is a colorless, odorless, poisonous gas, a product of incomplete burning of hydrocarbon-based fuels. Carbon monoxide consists of a single carbon atom and a single oxygen atom linked together (CO), the product of incomplete combustion of fuel. Most CO is produced when air-to-fuel ratios are too low in the engine during vehicle starting, when cars are not tuned properly, and at higher altitudes, where thin air reduces the amount of oxygen available for combustion. Two-thirds of the carbon monoxide emissions come from transportation sources, with the largest contribution coming from cars. In urban areas, the passenger vehicle contribution to carbon monoxide pollution can exceed 90%.
Carbon Dioxide U.S. Environmental Protection Agency (EPA) originally viewed carbon dioxide as a product of "perfect" combustion, but now views CO2 as a pollution concern. Carbon dioxide is a greenhouse gas that traps the earth's heat and contributes to Climate Change
Evaporative Emissions Hydrocarbon pollutants also escape into the air through fuel evaporation - evaporation causes significant hydrocarbon pollution from cars on hot days when ozone levels are highest. Evaporative emissions occur several ways:
Benzene is the main toxin in the hydrocarbon fraction of exhaust. Benzene and other less known hydrocarbons are produced in petroleum refining, and are widely used as solvents and as materials in the production of various industrial products and pesticides. Benzene also is found in gasoline and in cigarette smoke. Other environmental sources of benzene include gasoline (filling) stations, underground storage tanks that leak, wastewater from industries that use benzene, chemical spills, and groundwater next to landfills containing benzene. Exposure to benzene can cause cancer, especially leukemias and lymphomas. Benzene has a suppressive effect on bone marrow and it impairs blood cell maturation and amplification.
Vaccinationshttp://www.naturalnews.com/034012_vacinations_dangers.html, http://truedemocracyparty.net/2012/01/deadly-vaccines-death-serum/
Cancerhttp://tbyil.com/waroncancer.htm, http://cancertruth.org/Victom-Cancer-Conspiracy.htm, http://www.naturalnews.com/024633_cell_phone_health_WHO.html, http://www.salem-news.com/articles/may092010/cancer-truth-tk.php , http://old.disinfo.com/archive/pages/dossier/id336/pg1/index.html
http://healthwyze.org/index.php/component/content/article/431-the-american-cancer-society-admits-that-cancers-go-away-naturally.htmlhttp://healthwyze.org/index.php/component/content/article/419-a-new-method-of-poisoning-us-with-radiation-high-efficiency-light-bulbs.html
Posted by Armand RossettiSeptember 17, 2008 http://westpalmbeach.injuryboard.com/medical-devices-and-implants/gadolinium-is-linked-to-a-new-disease-with-no-known-treatment.aspx?googleid=247660
The relatively new medical device is gadolinium-based contrast agent (GBCA or gadolinium) used during certain MRI or similar procedures. The new kidney related disease that is little more than a decade old is called Nephrogenic Systemic Fibrosis (NSF). Caretakers inject the GBCA into the bloodstream just before patients undergo certain types of Magnetic Resonance Imaging (MRI).
Here is an example of how NSF might occur: Patients undergo an MRI, MRA, or CT scan. As part of the procedure, doctors inject the patient with a contrast solution containing a rare earth metal called gadolinium. There are different commercial names for gadolinium-based contrast marker: Omniscan (General Electric), Magnavist (Bayer Healthcare), OptiMark (Mallinckrodt), MultiHance (Bracco), and ProHance (Bracco).
After being injected, the Gadolinium dissipates throughout the body. Ordinarily, gadolinium is extremely toxic to human tissues, and manufacturers have to chelate or coat gadolinium with benign chemicals to make it safe for use as an internal marker. Once the chelated gadolinium disperses in the body, doctors can use MRI to obtain a sharper image than they might have obtained without using gadolinium.
Once the MRI procedure is complete, there is no longer a need for chelated gadolinium to be circulating in the bloodstream or to be bound to other body tissue, and the kidneys usually remove the coated gadolinium from the blood. However, patients who have poor kidney function have less capacity to remove gadolinium from the body. This incapacity to remove gadolinium at a preferred rate leads to persistently high levels of residual gadolinium in body tissue. Compromised kidneys’ lack the “speed” necessary to remove gadolinium, and this failure to eliminate the gadolinium leads to a more dangerous situation.
The chelated gadolinium that remains behind in the bodies of patients with poor kidney function then goes through a further process where the protective chelate breaks off of the gadolinium, and free, very toxic, gadolinium remains. Lack of kidney function permits greater amounts of free gadolinium to circulate in the body. And gadolinium toxicity then begins to manifest in vital organs located outside the circulatory system.
For example, after free gadolinium reaches the skin, it forms deposits that cause the skin to become less elastic, patchy and discolored. Skin changes include blackening of tissue and scarring, which deforms and hardens the skin. The hardening and deformation of the skin leads to substantial pain and loss of flexibility while moving the arms and walking. Muscle tissue also absorbs the toxic gadolinium, causing further pain and weakness, with notable hip involvement.
Free gadolinium also deposits in the eyes, and in vital organs, such as the lungs, heart, and liver. Gadolinium deposits in vital organs often result in fatal organ failure.
Once again symptoms of gadolinium poisoning include:
Hardening and darkening of the skin;
Dark patches on the skin;
Burning sensations;
Painful joints and stiffness;
Difficulty straightening the arms, legs and feet;
Weakness;
Yellow patches on the eyes; and
Hip pain.
Next we might ask why gadolinium escaped detection as a potential hazard.
Thyroid Problems by Dr. John Lowe
"I learned early during the last 16 years that the endocrinology specialty's judgment is corrupted by financial inducements from drug companies that profit from the TSH test and T4 replacement. All those years ago, my intention was to help revise the often harmful standard of care imposed by the endocrinology specialty for commercial rather than scientific reasons.
After careful consideration in the last several years, however, I realized that the specialty has its heels dug in; it's clear to me that the specialty won't volitionally rehabilitate itself into a rational, scientific, ethical, and respectable medical specialty.
Rather than rehabilitate, to this day, the specialty practices thuggery on a par with that of traditional organized crime. It does so by intimidating and persecuting clinicians who fail to cooperate in restricting their patients to T4 replacement—an often ineffective and harmful approach to therapy that's hugely profitable to Big Pharma and, by quid pro quo, to the specialty itself.
The well-intending persecuted clinicians are guilty of recognizing that T4 replacement doesn't work for and harms many patients. And they are guilty of having the courage to abide by the Hippocratic oath in using thyroid hormone therapies that get their patients well.
The thuggery of the specialty is in highly active gear. We know this because regularly, clinicians contact us and tell us of actions being taken against them by medical regulatory boards. Invariably, the action involves testimony against the clinicians by members of the endocrinology specialty or affiliates of theirs.
And what is the ultimate consequence of these actions against so many well-intended clinicians? Patients who need safe and effective thyroid hormone therapy are restricted to T4 replacement—an approach that published studies clearly show to be ineffective and harmful for many patients.
It seems that most every community has members of the endocrinology specialty who function as thugs. They act as enforcers of the command that clinicians use only TSH testing and T4 products. These thug endocrinologists file complaints against noncompliant clinicians. Then they testify against the clinicians before regulatory boards and courts and walk away scot-free after giving scientifically-false testimony in courts of law and other legal venues.
Experience tells me that the endocrinology specialty won't relent—not until it's forced to do so, probably by class action law suits and by forcible complaints for violations of medical ethics to purveyors of medical regulatory boards. To me, the final solution lies in the education of patients, clinicians, and legislators; legislation to disempower the endocrinology specialty from further harm to patients, clinicians, and the public welfare; and litigation against the specialty and its corporation supporters. The force of unforgiving corruption by the specialty and its affiliates has long loomed over me and my medical colleagues. .. All best wishes, Dr. Lowe."
Nuclear Power Threats Wednesday, August 24, 2011 :: Staff infoZine
Washington D.C. - infoZine -Robert Alvarez ([email protected] ) is a former senior policy adviser to the U.S. Secretary of Energy and now a senior scholar at the Institute for Policy Studies. In June, Alvarez wrote a piece titled "America's Nuclear Spent-Fuel Time Bombs: Japan's nuclear disaster should serve as a wake-up call for the United States." link
He said earlier this afternoon: "The earthquake measuring 5.9 on the Richter scale just occurred less than a hour ago. Its epicenter was in Mineral, Virginia -- approximately ten miles from two nuclear power reactors at the North Anna site. According to statement by a representative of Dominion Power the two reactors were designed to withstand a 5.9 to 6.1 quake. The Nuclear Regulatory Commission ranked the North Anna Reactors as being 7th in the nation in terms of earthquake risks.
"It is not clear what damage might have been sustained at the nuclear site. The North Anna reactors are of the Westinghouse Pressurized Water design and went on line in 1979 and 1980 respectively. Since then the reactors have generated approximately 1,200 metric tons of nuclear spent fuel containing about 228,000 curies of highly radioactive materials -- among the largest concentrations of radioactivity in the United States.
"The spent fuel pools at North Anna contain four to five times more than their original designs intended. As in Japan, all U.S. power nuclear power plant spent fuel pools do not have steel-lined, concrete barriers that cover reactor vessels to prevent the escape of radioactivity. They are not required to have back-up generators to keep used fuel rods cool, if offsite power is lost. Even though they contain some of the largest concentrations of radioactivity on the planet, spent reactor fuel pools in the U.S. are mostly contained in ordinary industrial structures designed to protect them against the elements. The North Anna reactors may have large cavities beneath their pools which could exacerbate leakage.
"Nearly 40 percent of the radioactivity in the North Anna spent fuel pools is cesium-137. They hold about 15 to 30 times more Cs-137 than was released by the Chernobyl accident in 1986. In 2003, my colleagues and I issued a study that warned that drainage of a pool might cause a catastrophic radiation fire, which could render an area uninhabitable greater than that created by the Chernobyl accident. A year later the National Academy of Sciences confirmed our findings and warned that “...partially or completely a spent fuel pool could lead to a propagating zirconium cladding fire and release large quantities of radioactive materials to the environment. ... Such fires would create thermal plumes that could potentially transport radioactive aerosols hundreds of miles downwind under appropriate atmospheric conditions.
Drug Firms Told to Pay $162M in Hepatitis C Case 10-10-2011” LAS VEGAS (AP)
— A Nevada jury on Monday ordered three pharmaceutical companies to pay $162.5 million in punitive damages in a lawsuit that accused them of negligently distributing large vials of an anesthetic to Las Vegas clinics at the center of a 2008 hepatitis C outbreak.
The damages awarded in Clark County District Court are on top of the $20.1 million in compensatory damages awarded to five plaintiffs Thursday after a jury found Teva Parenteral Medicines Inc., Baxter Healthcare Corp. and McKesson Corp. liable.
Plaintiffs' lawyers had accused the companies of putting corporate profits ahead of patient safety, and of recklessly distributing 50 milliliter vials of the powerful anesthetic Propofol to clinics where 10 or 20 milliliter doses were commonly needed for outpatient colonoscopy procedures. They had sought $600 million in punitive damages.
Teva attorney Mark Tully declined comment to reporters after the verdict was read, while photographers and news cameras snapped pictures of family members of the plaintiffs hugging each other and their lawyers.
Philip Hymanson, attorney for Baxter and McKesson, told jurors the Propofol was manufactured properly and delivered properly, and that clinic doctors and anesthesiologists were at fault if they misused it. Hymanson said there was no proof that happened. The companies maintain the vials were properly marked with instructions and warnings, and that jurors weren't allowed to hear that reusing syringes on multiple patients and not following proper sterilizing procedures could also have spread the incurable liver disease.
Teva was ordered to pay $89.4 million, while Baxter was told to pay $55.3 million and McKesson was ordered to pay $17.9 million.
The civil trials are the first of several now reaching trial phases in Las Vegas stemming from the 2008 hepatitis outbreak traced to colonoscopy clinics run by Dr. Dipak Desai. Southern Nevada health officials advised about 50,000 patients who received endoscopy procedures at Desai clinics to be tested for hepatitis. At least nine and as many as 114 patients were infected with the disease.
Another Clark County District Court jury last year found Teva and Baxter liable for damages in a similar case and awarded a combined $500 million in punitive damages to a Las Vegas private school principal and his wife. The man, Henry Chanin, claimed he contracted hepatitis C during a routine endoscopy procedure in 2006. Oskar Garcia can be reached on Twitter at http://twitter.com/oskargarcia
FDA Revokes Approval of Avastin for Breast Cancer WASHINGTON By LAURAN NEERGAARD
...the Food and Drug Administration said it appeared to be a false hope for breast cancer: Studies haven't found that it helps those patients live longer or brings enough other benefit to outweigh its dangerous side effects. "I did not come to this decision lightly," said the FDA's commissioner, Dr. Margaret Hamburg. But she said, "Sometimes despite the hopes of investigators, patients, industry and even the FDA itself, the results of rigorous testing can be disappointing."
Avastin remains on the market to treat certain colon, lung, kidney and brain cancers. Doctors are free to prescribe any marketed drug as they see fit. So even though the FDA formally revoked Avastin's approval as a breast cancer treatment, women could still receive it -- but their insurers may not pay for it. Some insurers already have quit in anticipation of FDA's long-expected ruling.
However, "Medicare will continue to cover Avastin," said Brian Cook, spokesman for the Centers for Medicare & Medicaid Services. The agency "will monitor the issue and evaluate coverage options as a result of action by the FDA but has no immediate plans to change coverage policies." Including infusion fees, a year's treatment with Avastin can reach $100,000....
The Avastin saga began in 2008, when an initial study suggested the drug could delay tumor growth for a few months in women whose breast cancer had spread to other parts of the body. Over the objection of its own advisers and to the surprise of cancer groups, FDA gave Avastin conditional approval -- it could be sold for such women while manufacturer Genentech tried to prove it really worked.
The problem: Ultimately, the tumor effect was even smaller than first thought. Across repeated studies, Avastin patients didn't live longer or have a higher quality of life. Yet the drug causes some life-threatening risks, including severe high blood pressure, massive bleeding, heart attack or heart failure and tears in the stomach and intestines, the FDA concluded. In two public hearings -- one last year and one this summer -- FDA advisers urged the agency to revoke that approval.
"The science is clear: Breast cancer patients are more likely to be harmed than helped by Avastin," said Diana Zuckerman of the National Research Center for Women and Families in Washington. Genentech had argued the drug should remain available while it conducted more research to see if certain subsets of breast cancer patients might benefit, and some patients and their doctors had argued passionately for the drug.
Petrochemicals in Our Lives from http://www.nutramed.com/environment/carsepa.htm
The Combustion Process Gasoline and diesel fuels are mixtures of hydrocarbons (made of hydrogen, oxygen and carbon atoms.) Hydrocarbons are burned by combining with oxygen. Nitrogen and sulphur atoms are also present and combine with oxygen when burned to produce gases. Automotive engines emit several types of pollutants.
Typical Engine Combustion:
Fuel + Air => Hydrocarbons + Nitrogen Oxides + Carbon Dioxide + Carbon Monoxide + water
Hydrocarbon emissions are fragments of fuel molecules, only partially burned. Hydrocarbons react in the presence of nitrogen oxides and sunlight to form ground-level ozone, a major component of smog. Ozone irritates the eyes, nose, throat and damages the lungs. A number of exhaust hydrocarbons are also toxic, some with the potential to cause cancer.
Nitrogen Oxides Under high pressure and temperature conditions in an engine, nitrogen and oxygen atoms react to form nitrogen oxides. Catalytic converters in car exhaust systems break down heavier nitrogen gases, forming nitrogen dioxide (NO2) - 300 times more potent than carbon dioxide as a greenhouse gas. NO2 makes up about 7.2 percent of the gases that cause global warming. Vehicles with catalytic converters produced nearly half of that NO2. NO2 also originates from nitrogen-based fertilizers and manure from farm animals.
Carbon Monoxide Carbon monoxide (CO) is a colorless, odorless, poisonous gas, a product of incomplete burning of hydrocarbon-based fuels. Carbon monoxide consists of a single carbon atom and a single oxygen atom linked together (CO), the product of incomplete combustion of fuel. Most CO is produced when air-to-fuel ratios are too low in the engine during vehicle starting, when cars are not tuned properly, and at higher altitudes, where thin air reduces the amount of oxygen available for combustion. Two-thirds of the carbon monoxide emissions come from transportation sources, with the largest contribution coming from cars. In urban areas, the passenger vehicle contribution to carbon monoxide pollution can exceed 90%.
Carbon Dioxide U.S. Environmental Protection Agency (EPA) originally viewed carbon dioxide as a product of "perfect" combustion, but now views CO2 as a pollution concern. Carbon dioxide is a greenhouse gas that traps the earth's heat and contributes to Climate Change
Evaporative Emissions Hydrocarbon pollutants also escape into the air through fuel evaporation - evaporation causes significant hydrocarbon pollution from cars on hot days when ozone levels are highest. Evaporative emissions occur several ways:
Benzene is the main toxin in the hydrocarbon fraction of exhaust. Benzene and other less known hydrocarbons are produced in petroleum refining, and are widely used as solvents and as materials in the production of various industrial products and pesticides. Benzene also is found in gasoline and in cigarette smoke. Other environmental sources of benzene include gasoline (filling) stations, underground storage tanks that leak, wastewater from industries that use benzene, chemical spills, and groundwater next to landfills containing benzene. Exposure to benzene can cause cancer, especially leukemias and lymphomas. Benzene has a suppressive effect on bone marrow and it impairs blood cell maturation and amplification.
Vaccinationshttp://www.naturalnews.com/034012_vacinations_dangers.html, http://truedemocracyparty.net/2012/01/deadly-vaccines-death-serum/
Cancerhttp://tbyil.com/waroncancer.htm, http://cancertruth.org/Victom-Cancer-Conspiracy.htm, http://www.naturalnews.com/024633_cell_phone_health_WHO.html, http://www.salem-news.com/articles/may092010/cancer-truth-tk.php , http://old.disinfo.com/archive/pages/dossier/id336/pg1/index.html
http://healthwyze.org/index.php/component/content/article/431-the-american-cancer-society-admits-that-cancers-go-away-naturally.htmlhttp://healthwyze.org/index.php/component/content/article/419-a-new-method-of-poisoning-us-with-radiation-high-efficiency-light-bulbs.html
